Abstract
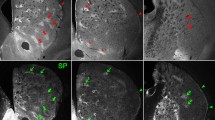
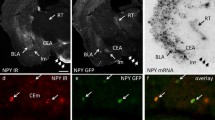
The striatum is divided into two compartments, the striosomes and extrastriosomal matrix, which differ in several cytochemical markers, input–output connections, and time of neurogenesis. Since it is thought that limbic, reward-related information and executive aspects of behavioral information may be differentially processed in the striosomes and matrix, respectively, intercompartmental communication should be of critical importance to proper functioning of the basal ganglia-thalamocortical circuits. Cholinergic interneurons are in a suitable position for this communication since they are preferentially located in the striosome-matrix boundaries and are known to elicit a conditioned pause response during sensorimotor learning. Recently, μ-opioid receptor (MOR) activation was found to presynaptically suppress the amplitude of GABAergic inhibitory postsynaptic currents in striosomal cells but not in matrix cells. Disinhibition of cells in the striosomes is further enhanced by inactivation of the protein kinase C cascade. We discuss in this review the possibility that MOR activation in the striosomes affects the activity of cholinergic interneurons and thus leads to changes in synaptic efficacy in the striatum.




Similar content being viewed by others
References
Gerdeman GL, Partridge JG, Lupica CR et al (2003) It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci 26(4):184–192
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Gerfen CR (1984) The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature 311(5985):461–464
Graybiel AM, Ragsdale CW Jr (1978) Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci U S A 75(11):5723–5726
Donoghue JP, Herkenham M (1986) Neostriatal projections from individual cortical fields conform to histochemically distinct striatal compartments in the rat. Brain Res 365(2):397–403
Eblen F, Graybiel AM (1995) Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. J Neurosci 15(9):5999–6013
Ragsdale CW Jr, Graybiel AM (1988) Fibers from the basolateral nucleus of the amygdala selectively innervate striosomes in the caudate nucleus of the cat. J Comp Neurol 269(4):506–522
Levesque M, Parent A (1998) Axonal arborization of corticostriatal and corticothalamic fibers arising from prelimbic cortex in the rat. Cereb Cort0ex 8(7):602–613
Wang H, Pickel VM (1998) Dendritic spines containing mu-opioid receptors in rat striatal patches receive asymmetric synapses from prefrontal corticostriatal afferents. J Comp Neurol 396(2):223–237
Bayer SA (1990) Neurogenetic patterns in the medial limbic cortex of the rat related to anatomical connections with the thalamus and striatum. Exp Neurol 107(2):132–142
Graybiel AM (1990) Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci 13(7):244–254
Brown LL, Feldman SM, Smith DM et al (2002) Differential metabolic activity in the striosome and matrix compartments of the rat striatum during natural behaviors. J Neurosci 22(1):305–314
White NM, Hiroi N (1998) Preferential localization of self-stimulation sites in striosomes/patches in the rat striatum. Proc Natl Acad Sci U S A 95(11):6486–6491
Miura M, Saino-Saito S, Masuda M et al (2007) Compartment-specific modulation of GABAergic synaptic transmission by mu-opioid receptor in the mouse striatum with green fluorescent protein-expressing dopamine islands. J Neurosci 27(36):9721–9728
Mansour A, Fox CA, Akil H et al (1995) Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci 18(1):22–29
Pert CB, Kuhar MJ, Snyder SH (1976) Opiate receptor: autoradiographic localization in rat brain. Proc Natl Acad Sci U S A 73(10):3729–3733
Herkenham M, Pert CB (1981) Mosaic distribution of opiate receptors, parafascicular projections and acetylcholinesterase in rat striatum. Nature 291(5814):415–418
Gerfen CR (1992) The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci 15:285–320
Delfs JM, Kong H, Mestek A et al (1994) Expression of mu opioid receptor mRNA in rat brain: an in situ hybridization study at the single cell level. J Comp Neurol 345(1):46–68
Kaneko T, Minami M, Satoh M et al (1995) Immunocytochemical localization of mu-opioid receptor in the rat caudate-putamen. Neurosci Lett 184(3):149–152
Wang H, Cuzon VC, Pickel VM (2003) Postnatal development of mu-opioid receptors in the rat caudate-putamen nucleus parallels asymmetric synapse formation. Neuroscience 118(3):695–708
Winzer-Serhan UH, Chen Y, Leslie FM (2003) Expression of opioid peptides and receptors in striatum and substantia nigra during rat brain development. J Chem Neuroanat 26(1):17–36
Martin-Schild S, Gerall AA, Kastin AJ et al (1999) Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol 405(4):450–471
Zadina JE, Hackler L, Ge LJ et al (1997) A potent and selective endogenous agonist for the mu-opiate receptor. Nature 386(6624):499–502
Arvidsson U, Riedl M, Chakrabarti S et al (1995) Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci 15(5 Pt 1):3328–3341
Graybiel AM, Ragsdale CW Jr, Yoneoka ES et al (1981) An immunohistochemical study of enkephalins and other neuropeptides in the striatum of the cat with evidence that the opiate peptides are arranged to form mosaic patterns in register with the striosomal compartments visible by acetylcholinesterase staining. Neuroscience 6(3):377–397
Wang H, Moriwaki A, Wang JB et al (1996) Ultrastructural immunocytochemical localization of mu opioid receptors and Leu5-enkephalin in the patch compartment of the rat caudate-putamen nucleus. J Comp Neurol 375(4):659–674
Wang H, Pickel VM (2001) Preferential cytoplasmic localization of delta-opioid receptors in rat striatal patches: comparison with plasmalemmal mu-opioid receptors. J Neurosci 21(9):3242–3250
Jiang ZG, North RA (1992) Pre- and postsynaptic inhibition by opioids in rat striatum. J Neurosci 12(1):356–361
Barral J, Mendoza E, Galarraga E et al (2003) The presynaptic modulation of corticostriatal afferents by mu-opioids is mediated by K+ conductances. Eur J Pharmacol 462(1–3):91–98
Fishell G, van der Kooy D (1987) Pattern formation in the striatum: developmental changes in the distribution of striatonigral neurons. J Neurosci 7(7):1969–1978
Moon Edley S, Herkenham M (1984) Comparative development of striatal opiate receptors and dopamine revealed by autoradiography and histofluorescence. Brain Res 305(1):27–42
Arlotta P, Molyneaux BJ, Jabaudon D et al (2008) Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J Neurosci 28(3):622–632
Janis LS, Cassidy RM, Kromer LF (1999) Ephrin-A binding and EphA receptor expression delineate the matrix compartment of the striatum. J Neurosci 19(12):4962–4971
Oo TF, Ries V, Cho J et al (2005) Anatomical basis of glial cell line-derived neurotrophic factor expression in the striatum and related basal ganglia during postnatal development of the rat. J Comp Neurol 484(1):57–67
Lopez-Martin E, Caruncho HJ, Rodriguez-Pallares J et al (1999) Striatal dopaminergic afferents concentrate in GDNF-positive patches during development and in developing intrastriatal striatal grafts. J Comp Neurol 406(2):199–206
Nishikawa S, Goto S, Yamada K et al (2003) Lack of Reelin causes malpositioning of nigral dopaminergic neurons: evidence from comparison of normal and Reln(rl) mutant mice. J Comp Neurol 461(2):166–173
van der Kooy D, Fishell G (1987) Neuronal birthdate underlies the development of striatal compartments. Brain Res 401(1):155–161
Krushel LA, Fishell G, van der Kooy D (1995) Pattern formation in the mammalian forebrain: striatal patch and matrix neurons intermix prior to compartment formation. Eur J Neurosci 7(6):1210–1219
Krushel LA, Connolly JA, van der Kooy D (1989) Pattern formation in the mammalian forebrain: patch neurons from the rat striatum selectively reassociate in vitro. Brain Res Dev Brain Res 47(1):137–142
Song DD, Harlan RE (1994) Genesis and migration patterns of neurons forming the patch and matrix compartments of the rat striatum. Brain Res Dev Brain Res 83(2):233–245
Matsushita N, Okada H, Yasoshima Y et al (2002) Dynamics of tyrosine hydroxylase promoter activity during midbrain dopaminergic neuron development. J Neurochem 82(2):295–304
Czubayko U, Plenz D (2002) Fast synaptic transmission between striatal spiny projection neurons. Proc Natl Acad Sci U S A 99(24):15764–15769
Tunstall MJ, Oorschot DE, Kean A et al (2002) Inhibitory interactions between spiny projection neurons in the rat striatum. J Neurophysiol 88(3):1263–1269
Venance L, Glowinski J, Giaume C (2004) Electrical and chemical transmission between striatal GABAergic output neurones in rat brain slices. J Physiol 559(Pt 1):215–230
Wilson CJ, Groves PM (1980) Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J Comp Neurol 194(3):599–615
Bolam JP, Somogyi P, Takagi H et al (1983) Localization of substance P-like immunoreactivity in neurons and nerve terminals in the neostriatum of the rat: a correlated light and electron microscopic study. J Neurocytol 12(2):325–344
Fujiyama F, Fritschy JM, Stephenson FA et al (2000) Synaptic localization of GABA(A) receptor subunits in the striatum of the rat. J Comp Neurol 416(2):158–172
Jaeger D, Kita H, Wilson CJ (1994) Surround inhibition among projection neurons is weak or nonexistent in the rat neostriatum. J Neurophysiol 72(5):2555–2558
Plenz D (2003) When inhibition goes incognito: feedback interaction between spiny projection neurons in striatal function. Trends Neurosci 26(8):436–443
Kita H, Kita T (2001) Number, origins, and chemical types of rat pallidostriatal projection neurons. J Comp Neurol 437(4):438–448
Finnegan TF, Chen SR, Pan HL (2006) Mu opioid receptor activation inhibits GABAergic inputs to basolateral amygdala neurons through Kv1.1/1.2 channels. J Neurophysiol 95(4):2032–2041
Endo K, Yawo H (2000) mu-Opioid receptor inhibits N-type Ca2+ channels in the calyx presynaptic terminal of the embryonic chick ciliary ganglion. J Physiol 524(Pt 3):769–781
Cherubini E, North RA (1985) Mu and kappa opioids inhibit transmitter release by different mechanisms. Proc Natl Acad Sci U S A 82(6):1860–1863
Stefani A, Surmeier DJ, Bernardi G (1994) Opioids decrease high-voltage activated calcium currents in acutely dissociated neostriatal neurons. Brain Res 642(1–2):339–343
Zhu W, Pan ZZ (2005) Mu-opioid-mediated inhibition of glutamate synaptic transmission in rat central amygdala neurons. Neuroscience 133(1):97–103
Vaughan CW, Ingram SL, Connor MA et al (1997) How opioids inhibit GABA-mediated neurotransmission. Nature 390(6660):611–614
Miura M, Watanabe M, Offermanns S et al (2002) Group I metabotropic glutamate receptor signaling via Galpha q/Galpha 11 secures the induction of long-term potentiation in the hippocampal area CA1. J Neurosci 22(19):8379–8390
Tanabe M, Kino Y, Honda M et al (2006) Presynaptic I1-imidazoline receptors reduce GABAergic synaptic transmission in striatal medium spiny neurons. J Neurosci 26(6):1795–1802
Chen XK, Wang LC, Zhou Y et al (2005) Activation of GPCRs modulates quantal size in chromaffin cells through G(betagamma) and PKC. Nat Neurosci 8(9):1160–1168
Ferre S, Ciruela F, Woods AS et al (2007) Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends Neurosci 30(9):440–446
Bailey CP, Smith FL, Kelly E et al (2006) How important is protein kinase C in mu-opioid receptor desensitization and morphine tolerance? Trends Pharmacol Sci 27(11):558–565
Walker RH, Arbuthnott GW, Baughman RW et al (1993) Dendritic domains of medium spiny neurons in the primate striatum: relationships to striosomal borders. J Comp Neurol 337(4):614–628
Kawaguchi Y, Wilson CJ, Emson PC (1989) Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol 62(5):1052–1068
Penny GR, Wilson CJ, Kitai ST (1988) Relationship of the axonal and dendritic geometry of spiny projection neurons to the compartmental organization of the neostriatum. J Comp Neurol 269(2):275–289
Bernacer J, Prensa L, Gimenez-Amaya JM (2007) Cholinergic interneurons are differentially distributed in the human striatum. PLoS ONE 2(11):e1174
Holt DJ, Graybiel AM, Saper CB (1997) Neurochemical architecture of the human striatum. J Comp Neurol 384(1):1–25
Holt DJ, Hersh LB, Saper CB (1996) Cholinergic innervation in the human striatum: a three-compartment model. Neuroscience 74(1):67–87
Kowall NW, Ferrante RJ, Beal MF et al (1987) Neuropeptide Y, somatostatin, and reduced nicotinamide adenine dinucleotide phosphate diaphorase in the human striatum: a combined immunocytochemical and enzyme histochemical study. Neuroscience 20(3):817–828
Faull RL, Dragunow M, Villiger JW (1989) The distribution of neurotensin receptors and acetylcholinesterase in the human caudate nucleus: evidence for the existence of a third neurochemical compartment. Brain Res 488(1–2):381–386
Jakab RL, Hazrati LN, Goldman-Rakic P (1996) Distribution and neurochemical character of substance P receptor (SPR)-immunoreactive striatal neurons of the macaque monkey: accumulation of SP fibers and SPR neurons and dendrites in "striocapsules" encircling striosomes. J Comp Neurol 369(1):137–149
Prensa L, Gimenez-Amaya JM, Parent A (1999) Chemical heterogeneity of the striosomal compartment in the human striatum. J Comp Neurol 413(4):603–618
Tepper JM, Koos T, Wilson CJ (2004) GABAergic microcircuits in the neostriatum. Trends Neurosci 27(11):662–669
Aosaki T, Kimura M, Graybiel AM (1995) Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J Neurophysiol 73(3):1234–1252
Graybiel AM, Baughman RW, Eckenstein F (1986) Cholinergic neuropil of the striatum observes striosomal boundaries. Nature 323(6089):625–627
Kubota Y, Kawaguchi Y (1993) Spatial distributions of chemically identified intrinsic neurons in relation to patch and matrix compartments of rat neostriatum. J Comp Neurol 332(4):499–513
Kawaguchi Y, Wilson CJ, Augood SJ et al (1995) Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci 18(12):527–535
Aosaki T, Kawaguchi Y (1996) Actions of substance P on rat neostriatal neurons in vitro. J Neurosci 16(16):5141–5153
Bolam JP, Ingham CA, Izzo PN et al (1986) Substance P-containing terminals in synaptic contact with cholinergic neurons in the neostriatum and basal forebrain: a double immunocytochemical study in the rat. Brain Res 397(2):279–289
Kuramoto E, Fujiyama F, Unzai T et al (2007) Metabotropic glutamate receptor 4-immunopositive terminals of medium-sized spiny neurons selectively form synapses with cholinergic interneurons in the rat neostriatum. J Comp Neurol 500(5):908–922
Liu H, Brown JL, Jasmin L et al (1994) Synaptic relationship between substance P and the substance P receptor: light and electron microscopic characterization of the mismatch between neuropeptides and their receptors. Proc Natl Acad Sci U S A 91(3):1009–1013
Miura M, Saino-Saito S, Masuda M et al (2007) Modulation by mu-opioid receptors on the excitability of cholinergic interneuron in the striosome/matrix compartment of the striatum. Soc Neurosci Abstr 514.16/SS30
Stanford IM, Cooper AJ (1999) Presynaptic mu and delta opioid receptor modulation of GABAA IPSCs in the rat globus pallidus in vitro. J Neurosci 19(12):4796–4803
Pakhotin P, Bracci E (2007) Cholinergic interneurons control the excitatory input to the striatum. J Neurosci 27(2):391–400
Miura M, Ishii K, Aosaki T et al (2006) Chronic nicotine treatment increases GABAergic input to striatal neurons. Neuroreport 17(5):537–540
Shen W, Hamilton SE, Nathanson NM et al (2005) Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci 25(32):7449–7458
Perez-Rosello T, Figueroa A, Salgado H et al (2005) Cholinergic control of firing pattern and neurotransmission in rat neostriatal projection neurons: role of CaV2.1 and CaV2.2 Ca2+ channels. J Neurophysiol 93(5):2507–2519
Koos T, Tepper JM (2002) Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci 22(2):529–535
Jabourian M, Venance L, Bourgoin S et al (2005) Functional mu opioid receptors are expressed in cholinergic interneurons of the rat dorsal striatum: territorial specificity and diurnal variation. Eur J Neurosci 21(12):3301–3309
Perez S, Tierney A, Deniau JM et al (2007) Tachykinin regulation of cholinergic transmission in the limbic/prefrontal territory of the rat dorsal striatum: implication of new neurokinine 1-sensitive receptor binding site and interaction with enkephalin/mu opioid receptor transmission. J Neurochem 103(6):2153–2163
Kimura M, Rajkowski J, Evarts E (1984) Tonically discharging putamen neurons exhibit set-dependent responses. Proc Natl Acad Sci U S A 81(15):4998–5001
Wilson CJ (2005) The mechanism of intrinsic amplification of hyperpolarizations and spontaneous bursting in striatal cholinergic interneurons. Neuron 45(4):575–585
Aosaki T, Tsubokawa H, Ishida A et al (1994) Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci 14(6):3969–3984
Morris G, Arkadir D, Nevet A et al (2004) Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron 43(1):133–143
Rice ME, Cragg SJ (2004) Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci 7(6):583–584
Zhang H, Sulzer D (2004) Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci 7(6):581–582
Cragg SJ (2006) Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci 29(3):125–131
Aosaki T, Graybiel AM, Kimura M (1994) Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science 265(5170):412–415
Suzuki T, Miura M, Nishimura K et al (2001) Dopamine-dependent synaptic plasticity in the striatal cholinergic interneurons. J Neurosci 21(17):6492–6501
Reynolds JN, Hyland BI, Wickens JR (2004) Modulation of an afterhyperpolarization by the substantia nigra induces pauses in the tonic firing of striatal cholinergic interneurons. J Neurosci 24(44):9870–9877
Deng P, Zhang Y, Xu ZC (2007) Involvement of I(h) in dopamine modulation of tonic firing in striatal cholinergic interneurons. J Neurosci 27(12):3148–3156
Maurice N, Mercer J, Chan CS et al (2004) D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J Neurosci 24(46):10289–10301
Wang Z, Kai L, Day M et al (2006) Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron 50(3):443–452
Ferry AT, Ongur D, An X et al (2000) Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. J Comp Neurol 425(3):447–470
Graybiel AM, Saka E (2002) A genetic basis for obsessive grooming. Neuron 33(1):1–2
Graybiel AM, Rauch SL (2000) Toward a neurobiology of obsessive-compulsive disorder. Neuron 28(2):343–347
Graybiel AM, Canales JJ (2001) The neurobiology of repetitive behaviors: clues to the neurobiology of Tourette syndrome. Adv Neurol 85:123–131
Pisani A, Bernardi G, Ding J et al (2007) Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci 30(10):545–553
The Huntington’s Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72(6):971–983
Tippett LJ, Waldvogel HJ, Thomas SJ et al (2007) Striosomes and mood dysfunction in Huntington’s disease. Brain 130(Pt 1):206–221
Lee LV, Maranon E, Demaisip C et al (2002) The natural history of sex-linked recessive dystonia parkinsonism of Panay, Philippines (XDP). Parkinsonism Relat Disord 9(1):29–38
Lee LV, Pascasio FM, Fuentes FD et al (1976) Torsion dystonia in Panay, Philippines. Adv Neurol 14:137–151
Kaji R, Goto S, Tamiya G et al (2005) Molecular and anatomical bases of dystonia: X-linked recessive dystonia-parkinsonism (DYT3). Rinsho Shinkeigaku 45(11):811–814
Kaji R, Goto S, Tamiya G et al (2005) Molecular dissection and anatomical basis of dystonia: X-linked recessive dystonia-parkinsonism (DYT3). J Med Invest 52(Suppl):280–283
Goto S, Lee LV, Munoz EL et al (2005) Functional anatomy of the basal ganglia in X-linked recessive dystonia-parkinsonism. Ann Neurol 58(1):7–17
Canales JJ, Graybiel AM (2000) A measure of striatal function predicts motor stereotypy. Nat Neurosci 3(4):377–383
Capper-Loup C, Canales JJ, Kadaba N et al (2002) Concurrent activation of dopamine D1 and D2 receptors is required to evoke neural and behavioral phenotypes of cocaine sensitization. J Neurosci 22(14):6218–6227
Saka E, Goodrich C, Harlan P et al (2004) Repetitive behaviors in monkeys are linked to specific striatal activation patterns. J Neurosci 24(34):7557–7565
Vanderschuren LJ, Schoffelmeer AN, Van Leeuwen SD et al (2002) Compartment-specific changes in striatal neuronal activity during expression of amphetamine sensitization are the result of drug hypersensitivity. Eur J Neurosci 16(12):2462–2468
Glickstein SB, Schmauss C (2004) Effect of methamphetamine on cognition and repetitive motor behavior of mice deficient for dopamine D2 and D3 receptors. Ann N Y Acad Sci 1025:110–118
Saka E, Iadarola M, Fitzgerald DJ et al (2002) Local circuit neurons in the striatum regulate neural and behavioral responses to dopaminergic stimulation. Proc Natl Acad Sci U S A 99(13):9004–9009
Balcells-Olivero M, Vezina P (1997) Effects of naltrexone on amphetamine-induced locomotion and rearing: acute and repeated injections. Psychopharmacology (Berl) 131(3):230–238
Diaz-Otanez CS, Capriles NR, Cancela LM (1997) D1 and D2 dopamine and opiate receptors are involved in the restraint stress-induced sensitization to the psychostimulant effects of amphetamine. Pharmacol Biochem Behav 58(1):9–14
Heidbreder C, Goldberg SR, Shippenberg TS (1993) Inhibition of cocaine-induced sensitization by the delta-opioid receptor antagonist naltrindole. Eur J Pharmacol 243(2):123–127
Hooks MS, Jones DN, Justice JB Jr et al (1992) Naloxone reduces amphetamine-induced stimulation of locomotor activity and in vivo dopamine release in the striatum and nucleus accumbens. Pharmacol Biochem Behav 42(4):765–770
Jones DN, Bowen WD, Portoghese PS et al (1993) Delta-opioid receptor antagonists attenuate motor activity induced by amphetamine but not cocaine. Eur J Pharmacol 249(2):167–177
Jones DN, Holtzman SG (1994) Influence of naloxone upon motor activity induced by psychomotor stimulant drugs. Psychopharmacology (Berl) 114(2):215–224
Kuzmin AV, Gerrits MA, van Ree JM et al (1997) Naloxone inhibits the reinforcing and motivational aspects of cocaine addiction in mice. Life Sci 60(18):PL-257–PL-264
Sala M, Braida D, Colombo M et al (1995) Behavioral and biochemical evidence of opioidergic involvement in cocaine sensitization. J Pharmacol Exp Ther 274(1):450–457
Schad CA, Justice JB Jr, Holtzman SG (1996) Differential effects of delta- and mu-opioid receptor antagonists on the amphetamine-induced increase in extracellular dopamine in striatum and nucleus accumbens. J Neurochem 67(6):2292–2299
Schad CA, Justice JB Jr, Holtzman SG (1995) Naloxone reduces the neurochemical and behavioral effects of amphetamine but not those of cocaine. Eur J Pharmacol 275(1):9–16
Woo SK, Hitzemann RJ, Loh HH (1985) Specific opioid-amphetamine interactions in the caudate putamen. Psychopharmacology (Berl) 85(3):371–376
Horner KA, Keefe KA (2006) Regulation of psychostimulant-induced preprodynorphin, c-fos and zif/268 messenger RNA expression in the rat dorsal striatum by mu opioid receptor blockade. Eur J Pharmacol 532(1–2):61–73
Kawaguchi Y (1992) Large aspiny cells in the matrix of the rat neostriatum in vitro: physiological identification, relation to the compartments and excitatory postsynaptic currents. J Neurophysiol 67(6):1669–1682
Acknowledgement
This work was supported by grants from the Ministry of Education, Science, Sports, Culture, and Technology of Japan, and from the Smoking Research Foundation of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miura, M., Masuda, M. & Aosaki, T. Roles of μ-Opioid Receptors in GABAergic Synaptic Transmission in the Striosome and Matrix Compartments of the Striatum. Mol Neurobiol 37, 104–115 (2008). https://doi.org/10.1007/s12035-008-8023-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-008-8023-2