Abstract
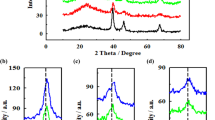
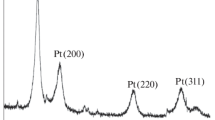
A novel polymer–carbon (PTh–C) nanocomposites containing different percentages of polythiophene (10, 20 and 50% (w/w)) and carbon (Vulcan XC-72) was prepared by a facile solution dispersion method and used to support platinum nanoparticles. The effect of using different percentages of polythiophene in nanocomposites and subsequently prepared electrocatalysts was investigated. The resultant electrocatalysts were extensively characterized by physical (X-ray diffraction (XRD) and transmission electron microscopy (TEM)) and electrochemical (cyclic voltammetry (CV)) techniques. The TEM results showed that the fine Pt nanoparticles prepared by ethylene glycol (EG) method were distributed on the surface of the 50% PTh–C nanocomposites successfully. From the XRD patterns, the average size of dispersed Pt nanoparticles with the face-centered cubic (fcc) structure on 50% PTh–C, 20% PTh–C, 10% PTh–C and carbon were about 4.9, 5.2, 5.4 and 6.1 nm, respectively. The conductivity of PTh–C with different percentages of pure PTh was compared with the conductivity of the corresponding support of pure PTh. It is observed that the conductivity of 50% PTh–C nanocomposites is about 600 times higher than that of pure PTh. Finally, CV measurements of hydrogen and methanol oxidations indicated that Pt/50% PTh–C had a higher electrochemical surface area and higher catalytic activity for methanol oxidation reaction compared to other electrocatalysts. These measurements showed that the Pt/50% PTh–C electrocatalyst by the value of 3.85 had higher \(I_{\mathrm{f}}/I_{\mathrm{b}}\) ratio with respect to Pt/10% PTh–C and Pt/20% PTh–C by the values of 2.66 and 2.0, respectively.






Similar content being viewed by others
References
Carrette L, Friedrich K A and Stimming U 2000 Chem. Phys. Chem. 1 162
Wang Y, Chen K S, Mishler J, Cho S C and Adroher X C 2011 Appl. Energy 88 981
Ellis M W, Von Spakovsky M R and Nelson D J 2001 Proc. IEEE 89 1808
Chen Z, Xu L, Li W, Waje M and Yan Y 2006 Nanotechnology 17 5254
Smitha B, Sridhar S and Khan A 2005 J. Membr. Sci. 259 10
Prater K B 1996 J. Power Sources 61 105
Wang S, Jiang S P, White T, Guo J and Wang X 2009 J. Phys. Chem. C 113 18935
Shao Y, Zhang S, Wang C, Nie Z, Liu J, Wang Y et al 2010 J. Power Sources 195 4600
Chung C G, Kim L, Sung Y W, Lee J and Chung J S 2009 Int. J. Hydrog. Energy 34 8974
Yaldagard M, Jahanshahi M and Seghatoleslami N 2013 World J. Nano Sci. Eng. 3 121
Sharma S and Pollet B G 2012 J. Power Sources 208 96
Beden B, Léger J-M and Lamy C 1992 in: J O M Bockris, B E Conway and R E White (eds) Modern aspects of electrochemistry (New York: Plenum Press) p 97
Wang Y-J, Wilkinson D P and Zhang J 2011 Chem. Rev. 111 7625
Sharma S and Pollet B G 2012 J. Power Sources 208 96
Wang J, Yin G-P, Zhang J, Wang Z and Gao Y 2007 Electrochim. Acta 52 7042
Adhikari A, Radhakrishnan S and Patil R 2009 Synth. Met. 159 1682
Antolini E 2010 Appl. Catal. B: Environ. 100 413
Unni S M, Dhavale V M, Pillai V K and Kurungot S 2010 J. Phys. Chem. C 114 14654
Vedrine J C, Dufaux M, Naccache C and Imelik B 1978 J. Chem. Soc. Faraday Trans. 1: Phys. Chem. Condens. Phases 74 440
Biloul A, Coowar F, Contamin O, Scarbeck G, Savy M, Van den Ham D et al 1990 J. Electroanal. Chem. Interfac. Electrochem. 289 189
Zhou J, Zhou X, Sun X, Li R, Murphy M, Ding Z et al 2007 Chem. Phys. Lett. 437 229
Dicks A L 2006 J. Power Sources 156 128
Antolini E 2009 Appl. Catal. B: Environ. 88 1
Maillard F, Simonov P A and Savinova E R 2009 in: P Serp and J Figueiredo (eds) Carbon materials for catalysis (New York: John Wiley & Sons, Inc.) chapter 12 p 429
Hezarjaribi M, Jahanshahi M, Rahimpour A and Yaldagard M 2014 Appl. Surf. Sci. 295 144
Wallace G G, Teasdale P R, Spinks G M and Kane-Maguire L A 2008 Conductive electroactive polymers: intelligent polymer systems, third edn (New York: CRC Press)
Antolini E and Gonzalez E 2009 Appl. Catal. A: General 365 1
Roncali J 1992 Chem. Rev. 92 711
Kattimani J, Sankarappa T, Praveenkumar K, Ashwajeet J and Ramanna R 2014 Int. J. Adv. Res. Phys. Sci. 1 17
Lee J M, Lee S J, Jung Y J and Kim J H 2008 Curr. Appl. Phys. 8 659
Sulub R, Martinez-Millan W and Smit M A 2009 Int. J. Electrochem. Sci. 4 1015
Schopf G and Koßmehl G 1997 Adv. Polym. Sci. 129 3
Cao Y, Wang P and Qian R 1985 Die Makromolekulare Chemie 186 1093
Tsakova V 2008 J. Solid State Electrochem. 12 1421
Gomez-Romero P 2001 Adv. Mat. 13 163
Fischer H 2003 Mat. Sci. Eng: C 23 763
Roy S, Christensen P, Hamnett A, Thomas K and Trapp V 1996 J. Electrochem. Soc. 143 3073
Takada T, Nakahara M, Kumagai H and Sanada Y 1996 Carbon 34 1087
Kumar D and Sharma R 1998 Eur. Polym. J. 34 1053
Gangopadhyay R and De A 2000 Chem. Mat. 12 608
Lin R, Cao C, Zhang H, Huang H and Ma J 2012 Int. J. Hydrog. Energy 37 4648
Koponen U, Kumpulainen H, Bergelin M, Keskinen J, Peltonen T, Valkiainen M et al 2003 J. Power Sources 118 325
Acknowledgements
We wish to express our sincere gratitude to the Nanotechnology Research Institute of Babol University for its scientific and financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nasrollahzadeh, M., Jahanshahi, M., Yaldagard, M. et al. Synthesis, characterization and comparison of polythiophene–carbon nanocomposite materials as Pt electrocatalyst supports for fuel cell applications. Bull Mater Sci 41, 85 (2018). https://doi.org/10.1007/s12034-018-1599-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-018-1599-x