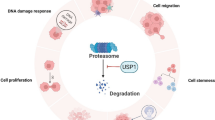
Abstract
p53 is a tumor suppressor gene activated in response to cellular stressors that inhibits cell cycle progression and induces pro-apoptotic signaling. The protein level of p53 is well balanced by the action of several E3 ligases and deubiquitinating enzymes (DUBs). Several DUBs have been reported to negatively regulate and promote p53 degradation in tumors. In this study, we identified USP19 as a negative regulator of p53 protein level. We demonstrate a direct interaction between USP19 and p53 by pull down assay. The overexpression of USP19 promoted ubiquitination of p53 and reduced its protein half-life. We also demonstrate that CRISPR/Cas9-mediated knockout of USP19 in cervical cancer cells elevates p53 protein levels, resulting in reduced colony formation, cell migration, and cell invasion. Overall, our results indicate that USP19 negatively regulates p53 protein levels in cervical cancer progression.





Similar content being viewed by others
Data Availability
Data availability is not applicable as no datasets were generated in this study.
References
Hager, K. M., & Gu, W. (2014). Understanding the non-canonical pathways involved in p53-mediated tumor suppression. Carcinogenesis, 35(4), 740–746. https://doi.org/10.1093/carcin/bgt487.
Hu, X., Chandler, J. D., Park, S., Liu, K., Fernandes, J., Orr, M., Smith, M. R., Ma, C., Kang, S. M., Uppal, K., Jones, D. P., & Go, Y. M. (2019). Low-dose cadmium disrupts mitochondrial citric acid cycle and lipid metabolism in mouse lung. Free radical biology & medicine, 131, 209–217. https://doi.org/10.1016/j.freeradbiomed.2018.12.005.
Kruiswijk, F., Labuschagne, C. F., & Vousden, K. H. (2015). p53 in survival, death and metabolic health: A lifeguard with a licence to kill. Nature reviews Molecular cell biology, 16(7), 393–405. https://doi.org/10.1038/nrm4007.
Song, H., Hollstein, M., & Xu, Y. (2007). p53 gain-of-function cancer mutants induce genetic instability by inactivating ATM. Nature cell biology, 9(5), 573–580. https://doi.org/10.1038/ncb1571.
Fearon, E. R., & Vogelstein, B. (1990). A genetic model for colorectal tumorigenesis. Cell, 61(5), 759–767. https://doi.org/10.1016/0092-8674(90)90186-i.
Malkin, D., Li, F. P., Strong, L. C., Fraumeni, J. F. Jr., Nelson, C. E., Kim, D. H., Kassel, J., Gryka, M. A., Bischoff, F. Z., Tainsky, M. A., et al. (1990). Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science (New York NY), 250(4985), 1233–1238. https://doi.org/10.1126/science.1978757.
Miller, L. D., Smeds, J., George, J., Vega, V. B., Vergara, L., Ploner, A., Pawitan, Y., Hall, P., Klaar, S., Liu, E. T., & Bergh, J. (2005). An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proceedings of the National Academy of Sciences of the United States of America, 102(38), 13550–13555. https://doi.org/10.1073/pnas.0506230102.
Olivier, M., & Taniere, P. (2011). Somatic mutations in cancer prognosis and prediction: Lessons from TP53 and EGFR genes. Current opinion in oncology, 23(1), 88–92. https://doi.org/10.1097/CCO.0b013e3283412dfa.
Rivlin, N., Brosh, R., Oren, M., & Rotter, V. (2011). Mutations in the p53 tumor suppressor gene: Important milestones at the various steps of Tumorigenesis. Genes & cancer, 2(4), 466–474. https://doi.org/10.1177/1947601911408889.
Yuan, J., Luo, K., Zhang, L., Cheville, J. C., & Lou, Z. (2010). USP10 regulates p53 localization and stability by deubiquitinating p53. Cell, 140(3), 384–396. https://doi.org/10.1016/j.cell.2009.12.032.
Pei, D., Zhang, Y., & Zheng, J. (2012). Regulation of p53: A collaboration between Mdm2 and mdmx. Oncotarget, 3(3), 228–235. https://doi.org/10.18632/oncotarget.443.
Wade, M., Wang, Y. V., & Wahl, G. M. (2010). The p53 orchestra: Mdm2 and Mdmx set the tone. Trends in cell biology, 20(5), 299–309. https://doi.org/10.1016/j.tcb.2010.01.009.
Li, Y., Ma, C., Zhou, T., Liu, Y., Sun, L., & Yu, Z. (2016). TRIM65 negatively regulates p53 through ubiquitination. Biochemical and biophysical research communications, 473(1), 278–282. https://doi.org/10.1016/j.bbrc.2016.03.093.
Freedman, D. A., & Levine, A. J. (1998). Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Molecular and cellular biology 18 (12):7288–7293. doi:https://doi.org/10.1128/mcb.18.12.7288.
Chène, P. (2003). Inhibiting the p53-MDM2 interaction: An important target for cancer therapy. Nature reviews Cancer, 3(2), 102–109. https://doi.org/10.1038/nrc991.
Sane, S., & Rezvani, K. (2017). Essential roles of E3 ubiquitin ligases in p53 regulation. International journal of molecular sciences, 18(2), https://doi.org/10.3390/ijms18020442.
Lim, S. K., Shin, J. M., Kim, Y. S., & Baek, K. H. (2004). Identification and characterization of murine mHAUSP encoding a deubiquitinating enzyme that regulates the status of p53 ubiquitination. International journal of oncology, 24(2), 357–364.
Cummins, J. M., Rago, C., Kohli, M., Kinzler, K. W., Lengauer, C., & Vogelstein, B. (2004). Tumour suppression: Disruption of HAUSP gene stabilizes p53. Nature, 428(6982), 1–pfollowing486. https://doi.org/10.1038/nature02501.
Ke, J. Y., Dai, C. J., Wu, W. L., Gao, J. H., Xia, A. J., Liu, G. P., Lv, K. S., & Wu, C. L. (2014). USP11 regulates p53 stability by deubiquitinating p53. Journal of Zhejiang University Science B, 15(12), 1032–1038. https://doi.org/10.1631/jzus.B1400180.
Liu, J., Chung, H. J., Vogt, M., Jin, Y., Malide, D., He, L., Dundr, M., & Levens, D. (2011). JTV1 co-activates FBP to induce USP29 transcription and stabilize p53 in response to oxidative stress. The EMBO journal, 30(5), 846–858. https://doi.org/10.1038/emboj.2011.11.
Hock, A. K., Vigneron, A. M., Carter, S., Ludwig, R. L., & Vousden, K. H. (2011). Regulation of p53 stability and function by the deubiquitinating enzyme USP42. The EMBO journal, 30(24), 4921–4930. https://doi.org/10.1038/emboj.2011.419.
Kwon, S. K., Saindane, M., & Baek, K. H. (2017). p53 stability is regulated by diverse deubiquitinating enzymes. Biochimica et biophysica acta reviews on cancer 1868 (2):404–411. doi:https://doi.org/10.1016/j.bbcan.2017.08.001.
Zou, Q., Jin, J., Hu, H., Li, H. S., Romano, S., Xiao, Y., Nakaya, M., Zhou, X., Cheng, X., Yang, P., Lozano, G., Zhu, C., Watowich, S. S., Ullrich, S. E., & Sun, S. C. (2014). USP15 stabilizes MDM2 to mediate cancer-cell survival and inhibit antitumor T cell responses. Nature immunology, 15(6), 562–570. https://doi.org/10.1038/ni.2885.
Zhang, X., Berger, F. G., Yang, J., & Lu, X. (2011). USP4 inhibits p53 through deubiquitinating and stabilizing ARF-BP1. The EMBO journal, 30(11), 2177–2189. https://doi.org/10.1038/emboj.2011.125.
Li, J., Wang, Y., Luo, Y., Liu, Y., Yi, Y., Li, J., Pan, Y., Li, W., You, W., Hu, Q., Zhao, Z., Zhang, Y., Cao, Y., Zhang, L., Yuan, J., & Xiao, Z. J. (2022). USP5-Beclin 1 axis overrides p53-dependent senescence and drives Kras-induced tumorigenicity. Nature communications, 13(1), 7799. https://doi.org/10.1038/s41467-022-35557-y.
Stevenson, L. F., Sparks, A., Allende-Vega, N., Xirodimas, D. P., Lane, D. P., & Saville, M. K. (2007). The deubiquitinating enzyme USP2a regulates the p53 pathway by targeting Mdm2. The EMBO journal, 26(4), 976–986. https://doi.org/10.1038/sj.emboj.7601567.
Lahav-Baratz, S., Kravtsova-Ivantsiv, Y., Golan, S., & Ciechanover, A. (2017). The testis-specific USP26 is a deubiquitinating enzyme of the ubiquitin ligase Mdm2. Biochemical and biophysical research communications 482 (1):106–111. doi:https://doi.org/10.1016/j.bbrc.2016.10.135.
Rossi, F. A., & Rossi, M. (2022). Emerging role of ubiquitin-specific protease 19 in oncogenesis and Cancer Development. Frontiers in cell and developmental biology, 10, 889166. https://doi.org/10.3389/fcell.2022.889166.
Hu, W., Su, Y., Fei, X., Wang, X., Zhang, G., Su, C., Du, T., Yang, T., Wang, G., Tang, Z., & Zhang, J. (2020). Ubiquitin specific peptidase 19 is a prognostic biomarker and affect the proliferation and migration of clear cell renal cell carcinoma. Oncology reports, 43(6), 1964–1974. https://doi.org/10.3892/or.2020.7565.
Kang, H., Choi, M. C., Kim, S., Jeong, J. Y., Kwon, A. Y., Kim, T. H., Kim, G., Joo, W. D., Park, H., Lee, C., Song, S. H., Jung, S. G., Hwang, S., & An, H. J. (2021). USP19 and RPL23 as candidate prognostic markers for Advanced-Stage High-Grade Serous Ovarian Carcinoma. Cancers, 13(16), https://doi.org/10.3390/cancers13163976.
Mei, Y., Hahn, A. A., Hu, S., & Yang, X. (2011). The USP19 deubiquitinase regulates the stability of c-IAP1 and c-IAP2. The Journal of biological chemistry, 286(41), 35380–35387. https://doi.org/10.1074/jbc.M111.282020.
Mirza, A., McGuirk, M., Hockenberry, T. N., Wu, Q., Ashar, H., Black, S., Wen, S. F., Wang, L., Kirschmeier, P., Bishop, W. R., Nielsen, L. L., Pickett, C. B., & Liu, S. (2002). Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway. Oncogene, 21(17), 2613–2622. https://doi.org/10.1038/sj.onc.1205353.
Wang, Z., Fukuda, S., & Pelus, L. M. (2004). Survivin regulates the p53 tumor suppressor gene family. Oncogene, 23(49), 8146–8153. https://doi.org/10.1038/sj.onc.1207992.
Cheng, L., Zhou, Z., Flesken-Nikitin, A., Toshkov, I. A., Wang, W., Camps, J., Ried, T., & Nikitin, A. Y. (2021). Correction to: Rb inactivation accelerates neoplastic growth and substitutes for recurrent amplification of cIAP1, cIAP2 and Yap1 in sporadic mammary carcinoma associated with p53 deficiency. Oncogene, 40(9), 1754. https://doi.org/10.1038/s41388-021-01647-2.
Ramakrishna, S., Kwaku Dad, A. B., Beloor, J., Gopalappa, R., Lee, S. K., & Kim, H. (2014). Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome research, 24(6), 1020–1027. https://doi.org/10.1101/gr.171264.113.
Suresh, B., Ramakrishna, S., Kim, Y. S., Kim, S. M., Kim, M. S., & Baek, K. H. (2010). Stability and function of mammalian lethal giant larvae-1 oncoprotein are regulated by the scaffolding protein RanBPM. The Journal of biological chemistry, 285(46), 35340–35349. https://doi.org/10.1074/jbc.M110.156836.
Suresh, B., Ramakrishna, S., & Kim, H. (2017). Cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA for genome editing. Methods in molecular biology. (Clifton NJ), 1507, 81–94. https://doi.org/10.1007/978-1-4939-6518-2_7.
Elledge, S. J. (1996). Cell cycle checkpoints: Preventing an identity crisis. Science (New York NY), 274(5293), 1664–1672. https://doi.org/10.1126/science.274.5293.1664.
Sionov, R. V., & Haupt, Y. (1999). The cellular response to p53: The decision between life and death. Oncogene, 18(45), 6145–6157. https://doi.org/10.1038/sj.onc.1203130.
McLean, D. E., Kearney, J., & Cawley, M. F. (1999). Environmentally responsive temperature instability in pediatric spinal cord injury. Spinal cord, 37(10), 705–709. https://doi.org/10.1038/sj.sc.3100888.
Vousden, K. H., & Lu, X. (2002). Live or let die: The cell’s response to p53. Nature reviews Cancer, 2(8), 594–604. https://doi.org/10.1038/nrc864.
Prives, C., & Hall, P. A. (1999). The p53 pathway. The Journal of pathology, 187(1), 112–126. https://doi.org/10.1002/(sici)1096-9896(199901)187:1<112::Aid-path250>3.0.Co;2-3.
Feki, A., & Irminger-Finger, I. (2004). Mutational spectrum of p53 mutations in primary breast and ovarian tumors. Critical reviews in oncology/hematology, 52(2), 103–116. https://doi.org/10.1016/j.critrevonc.2004.07.002.
Baker, S. J., Fearon, E. R., Nigro, J. M., Hamilton, S. R., Preisinger, A. C., Jessup, J. M., vanTuinen, P., Ledbetter, D. H., Barker, D. F., Nakamura, Y., White, R., & Vogelstein, B. (1989). Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science (New York NY), 244(4901), 217–221. https://doi.org/10.1126/science.2649981.
Nigro, J. M., Baker, S. J., Preisinger, A. C., Jessup, J. M., Hostetter, R., Cleary, K., Bigner, S. H., Davidson, N., Baylin, S., Devilee, P., et al. (1989). Mutations in the p53 gene occur in diverse human tumour types. Nature, 342(6250), 705–708. https://doi.org/10.1038/342705a0.
Takahashi, T., Nau, M. M., Chiba, I., Birrer, M. J., Rosenberg, R. K., Vinocour, M., Levitt, M., Pass, H., Gazdar, A. F., & Minna, J. D. (1989). p53: A frequent target for genetic abnormalities in lung cancer. Science (New York NY), 246(4929), 491–494. https://doi.org/10.1126/science.2554494.
Hollstein, M. C., Metcalf, R. A., Welsh, J. A., Montesano, R., & Harris, C. C. (1990). Frequent mutation of the p53 gene in human esophageal cancer. Proceedings of the National Academy of Sciences of the United States of America, 87(24), 9958–9961. https://doi.org/10.1073/pnas.87.24.9958.
Donehower, L. A., Harvey, M., Slagle, B. L., McArthur, M. J., Montgomery, C. A. Jr., Butel, J. S., & Bradley, A. (1992). Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature, 356(6366), 215–221. https://doi.org/10.1038/356215a0.
Clague, M. J., Coulson, J. M., & Urbé, S. (2012). Cellular functions of the DUBs. Journal of cell science, 125(Pt 2), 277–286. https://doi.org/10.1242/jcs.090985.
Amerik, A. Y., & Hochstrasser, M. (2004). Mechanism and function of deubiquitinating enzymes. Biochimica et biophysica acta, 1695(1–3), 189–207. https://doi.org/10.1016/j.bbamcr.2004.10.003.
Sun, S. C. (2008). Deubiquitylation and regulation of the immune response. Nature reviews Immunology, 8(7), 501–511. https://doi.org/10.1038/nri2337.
Sarodaya, N., Karapurkar, J., Kim, K. S., Hong, S. H., & Ramakrishna, S. (2020). The role of deubiquitinating enzymes in hematopoiesis and hematological malignancies. Cancers, 12(5), https://doi.org/10.3390/cancers12051103.
Ramakrishna, S., Suresh, B., & Baek, K. H. (2011). The role of deubiquitinating enzymes in apoptosis. Cellular and molecular life sciences: CMLS, 68(1), 15–26. https://doi.org/10.1007/s00018-010-0504-6.
Chandrasekaran, A. P., Kaushal, K., Park, C. H., Kim, K. S., & Ramakrishna, S. (2021). USP32 confers cancer cell resistance to YM155 via promoting ER-associated degradation of solute carrier protein SLC35F2. Theranostics, 11(20), 9752–9771. https://doi.org/10.7150/thno.63806.
Haq, S., Sarodaya, N., Karapurkar, J. K., Suresh, B., Jo, J. K., Singh, V., Bae, Y. S., Kim, K. S., & Ramakrishna, S. (2022). CYLD destabilizes NoxO1 protein by promoting ubiquitination and regulates prostate cancer progression. Cancer letters, 525, 146–157. https://doi.org/10.1016/j.canlet.2021.10.032.
Ashcroft, M., Kubbutat, M. H., & Vousden, K. H. (1999). Regulation of p53 function and stability by phosphorylation. Molecular and cellular biology, 19(3), 1751–1758. https://doi.org/10.1128/mcb.19.3.1751.
Tang, Y., Zhao, W., Chen, Y., Zhao, Y., & Gu, W. (2008). Acetylation is indispensable for p53 activation. Cell, 133(4), 612–626. https://doi.org/10.1016/j.cell.2008.03.025.
He, M., Zhou, Z., Shah, A. A., Zou, H., Tao, J., Chen, Q., & Wan, Y. (2016). The emerging role of deubiquitinating enzymes in genomic integrity, diseases, and therapeutics. Cell & bioscience, 6, 62. https://doi.org/10.1186/s13578-016-0127-1.
Li, M., Chen, D., Shiloh, A., Luo, J., Nikolaev, A. Y., Qin, J., & Gu, W. (2002). Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature, 416(6881), 648–653. https://doi.org/10.1038/nature737.
Cho, J., Park, J., Shin, S. C., Jang, M., Kim, J. H., Kim, E. E., & Song, E. J. (2020). USP47 promotes tumorigenesis by negative regulation of p53 through Deubiquitinating Ribosomal protein S2. Cancers, 12(5), https://doi.org/10.3390/cancers12051137.
Qi, S. M., Cheng, G., Cheng, X. D., Xu, Z., Xu, B., Zhang, W. D., & Qin, J. J. (2020). Targeting USP7-Mediated deubiquitination of MDM2/MDMX-p53 pathway for Cancer Therapy: Are we there yet? Frontiers in cell and developmental biology, 8, 233. https://doi.org/10.3389/fcell.2020.00233.
Sheng, Y., Saridakis, V., Sarkari, F., Duan, S., Wu, T., Arrowsmith, C. H., & Frappier, L. (2006). Molecular recognition of p53 and MDM2 by USP7/HAUSP. Nature structural & molecular biology, 13(3), 285–291. https://doi.org/10.1038/nsmb1067.
Vos, R. M., Altreuter, J., White, E. A., & Howley, P. M. (2009). The ubiquitin-specific peptidase USP15 regulates human papillomavirus type 16 E6 protein stability. Journal of virology, 83(17), 8885–8892. https://doi.org/10.1128/jvi.00605-09.
Yang, W., Rozan, L. M., McDonald, E. R. 3rd, Navaraj, A., Liu, J. J., Matthew, E. M., Wang, W., Dicker, D. T., & El-Deiry, W. S. (2007). CARPs are ubiquitin ligases that promote MDM2-independent p53 and phospho-p53ser20 degradation. The Journal of biological chemistry, 282(5), 3273–3281. https://doi.org/10.1074/jbc.M610793200.
Leng, R. P., Lin, Y., Ma, W., Wu, H., Lemmers, B., Chung, S., Parant, J. M., Lozano, G., Hakem, R., & Benchimol, S. (2003). Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell, 112(6), 779–791. https://doi.org/10.1016/s0092-8674(03)00193-4.
Lee, J. T., & Gu, W. (2010). The multiple levels of regulation by p53 ubiquitination. Cell death and differentiation, 17(1), 86–92. https://doi.org/10.1038/cdd.2009.77.
Tavana, O., & Gu, W. (2017). Modulation of the p53/MDM2 interplay by HAUSP inhibitors. Journal of molecular cell biology, 9(1), 45–52. https://doi.org/10.1093/jmcb/mjw049.
Li, M., Brooks, C. L., Kon, N., & Gu, W. (2004). A dynamic role of HAUSP in the p53-Mdm2 pathway. Molecular cell, 13(6), 879–886. https://doi.org/10.1016/s1097-2765(04)00157-1.
Dayal, S., Sparks, A., Jacob, J., Allende-Vega, N., Lane, D. P., & Saville, M. K. (2009). Suppression of the deubiquitinating enzyme USP5 causes the accumulation of unanchored polyubiquitin and the activation of p53. The Journal of biological chemistry, 284(8), 5030–5041. https://doi.org/10.1074/jbc.M805871200.
Altun, M., Zhao, B., Velasco, K., Liu, H., Hassink, G., Paschke, J., Pereira, T., & Lindsten, K. (2012). Ubiquitin-specific protease 19 (USP19) regulates hypoxia-inducible factor 1α (HIF-1α) during hypoxia. The Journal of biological chemistry, 287(3), 1962–1969. https://doi.org/10.1074/jbc.M111.305615.
Zhu, Y., Gu, L., Lin, X., Zhou, X., Lu, B., Liu, C., Li, Y., Prochownik, E. V., Karin, M., Wang, F., & Li, Y. (2023). P53 deficiency affects cholesterol esterification to exacerbate hepatocarcinogenesis. Hepatology (Baltimore Md), 77(5), 1499–1511. https://doi.org/10.1002/hep.32518.
Dong, Z., Guo, S., Wang, Y., Zhang, J., Luo, H., Zheng, G., Yang, D., Zhang, T., Yan, L., Song, L., Liu, K., Sun, Z., Meng, X., Zheng, Z., Zhang, J., & Zhao, Y. (2020). USP19 enhances MMP2/MMP9-Mediated tumorigenesis in gastric Cancer. OncoTargets and therapy, 13, 8495–8510. https://doi.org/10.2147/ott.S240543.
Zhu, Y., Gu, L., Lin, X., Zhou, X., Lu, B., Liu, C., Lei, C., Zhou, F., Zhao, Q., Prochownik, E. V., & Li, Y. (2021). USP19 exacerbates lipogenesis and colorectal carcinogenesis by stabilizing ME1. Cell reports, 37(13), 110174. https://doi.org/10.1016/j.celrep.2021.110174.
Chandrasekaran, A. P., Tyagi, A., Poondla, N., Sarodaya, N., Karapurkar, J. K., Kaushal, K., Park, C. H., Hong, S. H., Kim, K. S., & Ramakrishna, S. (2022). Dual role of deubiquitinating enzyme USP19 regulates mitotic progression and tumorigenesis by stabilizing survivin. Molecular therapy: the journal of the American Society of Gene Therapy, 30(11), 3414–3429. https://doi.org/10.1016/j.ymthe.2022.07.019.
Acknowledgements
We would like to thank all the members of the Suri Lab and KSK Lab. We would also like to thank Professor Ryu Seong Eon, Hanyang University, Seoul, South Korea for his help in recombinant protein purification.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grants (2021M3A9H3015389 and 2021R1I1A1A01052637), a Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korea government (Ministry of Science and ICT, Ministry of Health & Welfare) (22A0304L1-01) and Medical Research Center (2017R1A5A2015395), funded by the National Research Foundation of Korea (NRF) of the Ministry of Science, ICT and Future Planning, Korea.
Author information
Authors and Affiliations
Contributions
A.T., J.K.K, and S.R.K. designed the study. A.T, J.K.K, and J.C.C conducted experiment and analyzed and interpreted the data. A.T. and S.R.K. co-wrote the manuscript. J.C.C. conducted all revision experiment. B.S., N.S., A.M.A., K.K., S.H., A.P.C., S.D., and V.S. assisted A.T with the experiments. B.S., K.S.K., and S.R. procured financial support and reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors have no competing interests regarding the content of this manuscript to declare.
Ethics Approval
This study was approved by the Hanyang University Institutional Review Board (approval number HYI-17-137-10).
Consent to Participate
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tyagi, A., Karapurkar, J.K., Colaco, J.C. et al. USP19 Negatively Regulates p53 and Promotes Cervical Cancer Progression. Mol Biotechnol (2023). https://doi.org/10.1007/s12033-023-00814-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-023-00814-y