Abstract
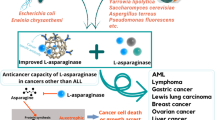
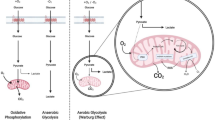
Asparagine is a non-essential amino acid crucial for protein biosynthesis and function, and therefore cell maintenance and growth. Furthermore, this amino acid has an important role in regulating several metabolic pathways, such as tricarboxylic acid cycle and the urea cycle. When compared to normal cells, tumor cells typically present a higher demand for asparagine, making it a compelling target for therapy. In this review article, we investigate different facets of asparagine bioavailability intricate role in malignant tumors raised from solid organs. We take a comprehensive look at asparagine synthetase expression and regulation in cancer, including the impact on tumor growth and metastasis. Moreover, we explore asparagine depletion through L-asparaginase as a potential therapeutic method for aggressive solid tumors, approaching different formulations of the enzyme and combinatory therapies. In summary, here we delve into studies about endogenous and exogenous asparagine availability in solid cancers, analyzing therapeutic implications and future challenges.


Similar content being viewed by others
Data availability
No datasets were generated or analyzed during the current study.
References
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022. https://doi.org/10.1158/2159-8290.CD-21-1059.
Navarro C, Ortega Á, Santeliz R, Garrido B, Chacín M, Galban N, et al. Metabolic reprogramming in cancer cells: emerging molecular mechanisms and novel therapeutic approaches. Pharmaceutics. 2022. https://doi.org/10.3390/pharmaceutics14061303.
Vettore L, Westbrook RL, Tennant DA. New aspects of amino acid metabolism in cancer. Br J Cancer. 2020. https://doi.org/10.1038/s41416-019-0620-5.
Knott SRV, Wagenblast E, Khan S, Kim SY, Soto M, Wagner M, et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature. 2018;554:378–81.
Pavlova NN, Hui S, Ghergurovich JM, Fan J, Intlekofer AM, White RM, et al. As extracellular glutamine levels decline, asparagine becomes an essential amino acid. Cell Metab. 2018;27:428-438.e5.
Lefin N, Miranda J, Beltrán JF, Belén LH, Effer B, Pessoa A, et al. Current state of molecular and metabolic strategies for the improvement of L-asparaginase expression in heterologous systems. Front Pharmacol. 2023.
Sun J, Nagel R, Zaal EA, Ugalde AP, Han R, Proost N, et al. SLC 1A3 contributes to L-asparaginase resistance in solid tumors. EMBO J. 2019. https://doi.org/10.15252/embj.2019102147.
Nakamura A, Nambu T, Ebara S, Hasegawa Y, Toyoshima K, Tsuchiya Y, et al. Inhibition of GCN2 sensitizes ASNS-low cancer cells to asparaginase by disrupting the amino acid response. Proc Natl Acad Sci USA. 2018. https://doi.org/10.1073/pnas.1805523115.
Pathria G, Lee JS, Hasnis E, Tandoc K, Scott DA, Verma S, et al. Translational reprogramming marks adaptation to asparagine restriction in cancer. Nat Cell Biol. 2019;21:1590.
Recouvreux MV, Grenier SF, Zhang Y, Esparza E, Lambies G, Galapate CM, et al. Glutamine mimicry suppresses tumor progression through asparagine metabolism in pancreatic ductal adenocarcinoma. Nat Cancer. 2023. https://doi.org/10.1038/s43018-023-00649-1.
Karpel-Massler G, Ramani D, Shu C, Halatsch ME, Westhoff MA, Bruce JN, et al. Metabolic reprogramming of glioblastoma cells by L-asparaginase sensitizes for apoptosis in vitro and in vivo. Oncotarget. 2016;7:33512.
Chandel NS. Amino acid metabolism. Cold Spring Harb Perspect Biol. 2021;13:a040584.
Kandasamy P, Zlobec I, Nydegger DT, Pujol-Giménez J, Bhardwaj R, Shirasawa S, et al. Oncogenic KRAS mutations enhance amino acid uptake by colorectal cancer cells via the hippo signaling effector YAP1. Mol Oncol. 2021;15:2782.
Taurino G, Chiu M, Bianchi MG, Griffini E, Bussolati O. The SLC38A5 /SNAT5 amino acid transporter: from pathophysiology to pro-cancer roles in the tumor microenvironment. Am J Physiol Cell Physiol. 2023;325:C550.
Magi S, Piccirillo S, Amoroso S, Lariccia V. Excitatory amino acid transporters (Eaats): glutamate transport and beyond. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20225674.
Krall AS, Xu S, Graeber TG, Braas D, Christofk HR. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat Commun. 2016. https://doi.org/10.1038/ncomms11457.
Luo M, Brooks M, Wicha MS. Asparagine and glutamine: co-conspirators fueling metastasis. Cell Metab. 2018;27:947–9. https://doi.org/10.1016/j.cmet.2018.04.012.
Zhang J, Fan J, Venneti S, Cross JR, Takagi T, Bhinder B, et al. Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Mol Cell. 2014;56:205.
Cui H, Darmanin S, Natsuisaka M, Kondo T, Asaka M, Shindoh M, et al. Enhanced expression of asparagine synthetase under glucose-deprived conditions protects pancreatic cancer cells from apoptosis induced by glucose deprivation and cisplatin. Cancer Res. 2007;67:3345.
Yang H, He X, Zheng Y, Feng W, Xia X, Yu X, et al. Down-regulation of asparagine synthetase induces cell cycle arrest and inhibits cell proliferation of breast cancer. Chem Biol Drug Des. 2014;84:578.
Xu Y, Lv F, Zhu X, Wu Y, Shen X. Loss of asparagine synthetase suppresses the growth of human lung cancer cells by arresting cell cycle at G0/G1 phase. Cancer Gene Ther. 2016;23:287.
Yu Q, Wang X, Wang L, Zheng J, Wang J, Wang B. Knockdown of asparagine synthetase (ASNS) suppresses cell proliferation and inhibits tumor growth in gastric cancer cells. Scand J Gastroenterol. 2016;51:1220.
Huang L, Guo Z, Wang F, Fu L. KRAS mutation: from undruggable to druggable in cancer. Signal Transduct Target Ther. 2021. https://doi.org/10.1038/s41392-021-00780-4.
Kerk SA, Papagiannakopoulos T, Shah YM, Lyssiotis CA. Metabolic networks in mutant KRAS-driven tumours: tissue specificities and the microenvironment. Nat Rev Cancer. 2021. https://doi.org/10.1038/s41568-021-00375-9.
Suzuki T, Kishikawa T, Sato T, Takeda N, Sugiura Y, Seimiya T, et al. Mutant KRAS drives metabolic reprogramming and autophagic flux in premalignant pancreatic cells. Cancer Gene Ther. 2022;29:505.
Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 1979;2009:325.
Chidley C, Darnell AM, Gaudio BL, Lien EC, Barbeau AM, Vander Heiden MG, et al. A CRISPRi/a screening platform to study cellular nutrient transport in diverse microenvironments. Nat Cell Biol. 2024;26:825–38. https://doi.org/10.1038/s41556-024-01402-1.
Toda K, Kawada K, Iwamoto M, Inamoto S, Sasazuki T, Shirasawa S, et al. Metabolic alterations caused by KRAS mutations in colorectal cancer contribute to cell adaptation to glutamine depletion by upregulation of asparagine synthetase. Neoplasia. 2016;18:654.
Gwinn DM, Lee AG, Briones-Martin-del-Campo M, Conn CS, Simpson DR, Scott AI, et al. Oncogenic KRAS regulates amino acid homeostasis and asparagine biosynthesis via ATF4 and Alters Sensitivity to L-Asparaginase. Cancer Cell. 2018;33:91.
Yang R, Li X, Wu Y, Zhang G, Liu X, Li Y, et al. EGFR activates GDH1 transcription to promote glutamine metabolism through MEK/ERK/ELK1 pathway in glioblastoma. Oncogene. 2020;39:2975.
Raho S, Capobianco L, Malivindi R, Vozza A, Piazzolla C, De Leonardis F, et al. KRAS-regulated glutamine metabolism requires UCP2-mediated aspartate transport to support pancreatic cancer growth. Nat Metab. 2020;2:1373.
Qin C, Yang X, Zhan Z. High expression of asparagine synthetase is associated with poor prognosis of breast cancer in chinese population. Cancer Biother Radiopharm. 2020. https://doi.org/10.1089/cbr.2019.3295.
Chiu M, Taurino G, Bianchi MG, Kilberg MS, Bussolati O. Asparagine synthetase in cancer: beyond acute lymphoblastic leukemia. Front Oncol. 2020. https://doi.org/10.3389/fonc.2019.01480.
Jiang J, Srivastava S, Seim G, Pavlova NN, King B, Zou L, et al. Promoter demethylation of the asparagine synthetase gene is required for ATF4-dependent adaptation to asparagine depletion. J Biol Chem. 2019;294:18674.
Liu WJ, Wang H, Peng XW, Da WW, Liu NW, Wang Y, et al. Asparagine synthetase expression is associated with the sensitivity to asparaginase in extranodal natural killer/T-cell lymphoma in vivo and in vitro. Onco Targets Ther. 2018;11:6605.
Dufour E, Gay F, Aguera K, Scoazec JY, Horand F, Lorenzi PL, et al. Pancreatic tumor sensitivity to plasma L-asparagine starvation. Pancreas. 2012;41:940.
Li H, Ning S, Ghandi M, Kryukov GV, Gopal S, Deik A, et al. The landscape of cancer cell line metabolism. Nat Med. 2019;25:850.
Li H, Zhou F, Du W, Dou J, Xu Y, Gao W, et al. Knockdown of asparagine synthetase by RNAi suppresses cell growth in human melanoma cells and epidermoid carcinoma cells. Biotechnol Appl Biochem. 2016;63:328.
Ueno T, Ohtawa K, Mitsui K, Kodera Y, Hiroto M, Matsushima A, et al. Cell cycle arrest and apoptosis of leukemia cells induced by L-asparaginase. Leukemia. 1997;11:1858.
Lubkowski J, Wlodawer A. Structural and biochemical properties of L-asparaginase. FEBS J. 2021;288:4183.
Kidd JG. Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum: I: course of transplanted cancers of various kinds in mice and rats given guinea pig serum, horse serum, or rabbit serum. J Exp Med. 1953;98:583.
Broome JD. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects: I: Properties of the L-asparaginase of guinea pig serum in relation to those of the antilymphoma substance. J Exp Med. 1963;118:121.
Yellin TO, Wriston JC. Purification and properties of guinea pig serum asparaginase. Biochemistry. 1966;5:1605.
Clarkson B, Krakoff I, Burchenal J, Karnofsky D, Golbey R, Dowling M, et al. Clinical results of treatment with E. coli L-asparaginase in adults with leukemia, lymphoma, and solid tumors. Cancer. 1970;25:279.
Strzelczyk P, Zhang D, Dyba M, Wlodawer A, Lubkowski J. Generalized enzymatic mechanism of catalysis by tetrameric l-asparaginases from mesophilic bacteria. Sci Rep. 2020. https://doi.org/10.1038/s41598-020-74480-4.
Chan WK, Lorenzi PL, Anishkin A, Purwaha P, Rogers DM, Sukharev S, et al. The glutaminase activity of L- Asparaginase is not required for anticancer activity against ASNS-negative cells. Blood. 2014;123:3596.
Chan WK, Horvath TD, Tan L, Link T, Harutyunyan KG, Pontikos MA, et al. Glutaminase activity of L-asparaginase contributes to durable preclinical activity against acute lymphoblastic leukemia. Mol Cancer Ther. 2019;18:1587.
Kamal N, Koh C, Samala N, Fontana RJ, Stolz A, Durazo F, et al. Asparaginase-induced hepatotoxicity: rapid development of cholestasis and hepatic steatosis. Hepatol Int. 2019;13:641.
Buddington RK, Buddington KK, Howard SC. Multiple asparaginase infusions cause increasingly severe acute hyperammonemia. Med Sci. 2022;10:43.
Tong WH, Rizzari C. Back to the future: the amazing journey of the therapeutic anti-leukemia enzyme asparaginase Erwinia chrysanthemi. Haematologic. 2023;108:2606.
Ashihara Y, Kono T, Yamazaki S, Inada Y. Modification of E. coli L-asparaginase with polyethylene glycol: disappearance of binding ability to anti-asparaginase serum. Biochem Biophys Res Commun. 1978;83:385.
Jiang J, Batra S, Zhang J. Asparagine: a metabolite to be targeted in cancers. Metabolites. 2021. https://doi.org/10.3390/metabo11060402.
Avramis VI, Sencer S, Periclou AP, Sather H, Bostrom BC, Cohen LJ, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood. 2002;99:1986.
Ho DH, Brown NS, Yen A, Holmes R, Keating M, Abuchowski A, et al. Clinical pharmacology of polyethylene glycol-L-asparaginase. Drug Metabolism and Disposition. 1986;14.
Pieters R, Hunger SP, Boos J, Rizzari C, Silverman L, Baruchel A, et al. L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer. 2011;117:238.
Narta UK, Kanwar SS, Azmi W. Pharmacological and clinical evaluation of l-asparaginase in the treatment of leukemia. Crit Rev Oncol Hematol. 2007;61:208–21.
Nguyen HA, Su Y, Lavie A. Design and characterization of erwinia chrysanthemi L-asparaginase variants with diminished L-glutaminase activity. J Biol Chem. 2016;291:17664.
Nguyen HA, Su Y, Zhang JY, Antanasijevic A, Caffrey M, Schalk AM, et al. A novel L-asparaginase with low L-glutaminase coactivity is highly efficacious against both T- and B-cell acute lymphoblastic Leukemias In Vivo. Cancer Res. 2018;78:1549.
Van Trimpont M, Schalk AM, De Visser Y, Nguyen HA, Reunes L, Vandemeulebroecke K, et al. In vivo stabilization of a less toxic asparaginase variant leads to a durable antitumor response in acute leukemia. Haematologica. 2023;108:409.
Silverman LB, Declerck L, Gelber RD, Kimball Dalton V, Asselin BL, Barr RD, et al. Results of Dana-Farber Institute Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1981–1995). Leukemia. 2000;14:2247.
O’Connell TM, Golzarri-Arroyo L, Pin F, Barreto R, Dickinson SL, Couch ME, et al. Metabolic biomarkers for the early detection of cancer cachexia. Front Cell Dev Biol. 2021. https://doi.org/10.3389/fcell.2021.720096.
Ni J, Zhang L. Cancer cachexia: definition, staging, and emerging treatments. Cancer Manag Res. 2020. https://doi.org/10.2147/CMAR.S261585.
Goodenough CG, Partin RE, Ness KK. Skeletal muscle and childhood cancer: where are we now and where we go from here. Aging Cancer. 2021;2.
Hochwald S, Heslin M. Plasma Amino Acid Concentrations in Cancer Cachexia. 1996.
Kunzke T, Buck A, Prade VM, Feuchtinger A, Prokopchuk O, Martignoni ME, et al. Derangements of amino acids in cachectic skeletal muscle are caused by mitochondrial dysfunction. J Cachexia Sarcopenia Muscle. 2020;11:226.
Bunpo P, Murray B, Cundiff J, Brizius E, Aldrich CJ, Anthony TG. Alanyl-glutamine consumption modifies the suppressive effect of L-asparaginase on lymphocyte populations in mice. J Nutr. 2008;138:338.
Ehsanipour EA, Sheng X, Behan JW, Wang X, Butturini A, Avramis VI, et al. Adipocytes cause leukemia cell resistance to l-asparaginase via release of glutamine. Cancer Res. 2013;73:2998.
van der Sluis IM, Vrooman LM, Pieters R, Baruchel A, Escherich G, Goulden N, et al. Consensus expert recommendations for identification and management of asparaginase hypersensitivity and silent inactivation. Haematologica. 2016;101:279.
Zhang B, Fan J, Zhang X, Shen W, Cao Z, Yang P, et al. Targeting asparagine and autophagy for pulmonary adenocarcinoma therapy. Appl Microbiol Biotechnol. 2016;100:9145.
Ji Y, Li L, Tao Q, Zhang X, Luan J, Zhao S, et al. Deprivation of asparagine triggers cytoprotective autophagy in laryngeal squamous cell carcinoma. Appl Microbiol Biotechnol. 2017;101:4951.
Chen Q, Ye L, Fan J, Zhang X, Wang H, Liao S, et al. Autophagy suppression potentiates the anti-glioblastoma effect of asparaginase in vitro and in vivo. Oncotarget. 2017;8:91052.
Wang Z, Xie Q, Zhou H, Zhang M, Shen J, Ju D. Amino acid degrading enzymes and autophagy in cancer therapy. Front Pharmacol. 2021. https://doi.org/10.3389/fphar.2020.582587.
Guo JY, White E. Autophagy, metabolism, and cancer. Cold Spring Harb Symp Quant Biol. 2016;81:73.
Monkkonen T, Debnath J. Inflammatory signaling cascades and autophagy in cancer. Autophagy. 2018. https://doi.org/10.1080/15548627.2017.1345412.
Kimmelman AC, White E. Autophagy and tumor metabolism. Cell Metab. 2017. https://doi.org/10.1016/j.cmet.2017.04.004.
Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29:2082.
Siu F, Bain PJ, Leblanc-Chaffin R, Chen H, Kilberg MS. ATF4 is a mediator of the nutrient-sensing response pathway that activates the human asparagine synthetase gene. J Biol Chem. 2002;277:24120.
Lopez CD, Kardosh A, Chen EY, Pegna GJ, Goodyear S, Taber E, et al. Casper: a phase I, open-label, dose finding study of calaspargase pegol-mnkl (cala) in combination with cobimetinib (cobi) in locally advanced or metastatic pancreatic ductal adenocarcinoma (PDAC). J Clin Oncol. 2023;41:TPS772.
Encarnación-Rosado J, Sohn ASW, Biancur DE, Lin EY, Osorio-Vasquez V, Rodrick T, et al. Targeting pancreatic cancer metabolic dependencies through glutamine antagonism. Nat Cancer. 2023. https://doi.org/10.1038/s43018-023-00647-3.
Haskell CM. L-Asparaginase; therapeutic and toxic effects in patients with neoplastic disease. N Engl J Med. 1969;281:1028.
Hays JL, Kim G, Walker A, Annunziata CM, Lee J-M, Squires J, et al. A phase II clinical trial of polyethylene glycol-conjugated L-asparaginase in patients with advanced ovarian cancer: Early closure for safety. Mol Clin Oncol. 2013;1:565.
Al-Dulimi AG, Al-Saffar AZ, Sulaiman GM, Khalil KAA, Khashan KS, Al-Shmgani HSA, et al. Immobilization of L-asparaginase on gold nanoparticles for novel drug delivery approach as anti-cancer agent against human breast carcinoma cells. J Mater Res Technol. 2020;9:15394.
Tam S-Y, Chung S-F, Kim C-F, To JC, So P-K, Cheung K-K, et al. Development of a bioengineered Erwinia chrysanthemi asparaginase to enhance its anti-solid tumor potential for treating gastric cancer. Int J Biol Macromol. 2023;253:127742.
Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021. https://doi.org/10.1038/s41568-021-00347-z.
Yang L, Chu Z, Liu M, Zou Q, Li J, Liu Q, et al. Amino acid metabolism in immune cells: essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy. J Hematol Oncol. 2023. https://doi.org/10.1186/s13045-023-01453-1.
Chen J, Cui L, Lu S, Xu S. Amino acid metabolism in tumor biology and therapy. Cell Death Dis. 2024. https://doi.org/10.1038/s41419-024-06435-w.
Wu J, Li G, Li L, Li D, Dong Z, Jiang P. Asparagine enhances LCK signalling to potentiate CD8+ T-cell activation and anti-tumour responses. Nat Cell Biol. 2021;23:75.
Hope HC, Brownlie RJ, Fife CM, Steele L, Lorger M, Salmond RJ. Coordination of asparagine uptake and asparagine synthetase expression modulates CD8+ T cell activation. JCI Insight. 2021. https://doi.org/10.1172/jci.insight.137761.
Gnanaprakasam JNR, Kushwaha B, Liu L, Chen X, Kang S, Wang T, et al. Asparagine restriction enhances CD8+ T cell metabolic fitness and antitumoral functionality through an NRF2-dependent stress response. Nat Metab. 2023;5:123.
Hersh EM. Immunosuppression by l-asparaginase and related enzymes: a review. Transplantation. 1971;12:368.
Kafkewitz D, Bendich A. Enzyme-induced asparagine and glutamine depletion and immune system function. Am J Clin Nutr. 1983. https://doi.org/10.1093/ajcn/37.6.1025.
Song P, Wang Z, Zhang X, Fan J, Li Y, Chen Q, et al. The role of autophagy in asparaginase-induced immune suppression of macrophages. Cell Death Dis. 2017;8:e2721.
Van Der Meer LT, Terry SYA, Van Ingen Schenau DS, Andree KC, Franssen GM, Roeleveld DM, et al. In vivo imaging of antileukemic drug asparaginase reveals a rapid macrophage-mediated clearance from the bone marrow. J Nuclear Med. 2017;58:214.
Nishikawa H, Koyama S. Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. J Immunother Cancer. 2021. https://doi.org/10.1136/jitc-2021-002591.
Peña-Romero AC, Orenes-Piñero E. Dual effect of immune cells within tumour microenvironment: pro-and anti-tumour effects and their triggers. Cancers. 2022. https://doi.org/10.3390/cancers14071681.
Shi H, Chi H. Metabolic control of treg cell stability, plasticity, and tissue-specific heterogeneity. Front Immunol. 2019. https://doi.org/10.3389/fimmu.2019.02716.
Bai J, Tang R, Zhou K, Chang J, Wang H, Zhang Q, et al. An asparagine metabolism-based classification reveals the metabolic and immune heterogeneity of hepatocellular carcinoma. BMC Med Genom. 2022. https://doi.org/10.1186/s12920-022-01380-z15.
Yu M, Henning R, Walker A, Kim G, Perroy A, Alessandro R, et al. L-asparaginase inhibits invasive and angiogenic activity and induces autophagy in ovarian cancer. J Cell Mol Med. 2012;16:2369.
Brumano LP, da Silva FVS, Costa-Silva TA, Apolinário AC, Santos JHPM, Kleingesinds EK, et al. Development of L-asparaginase biobetters: current research status and review of the desirable quality profiles. Front Bioeng Biotechnol. 2019;6:1–22.
Springer AD, Dowdy SF. GalNAc-siRNA Conjugates: leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid Ther. 2018. https://doi.org/10.1089/nat.2018.0736.
Nishikawa G, Kawada K, Hanada K, Maekawa H, Itatani Y, Miyoshi H, et al. Targeting asparagine synthetase in tumorgenicity using patient-derived tumor-initiating cells. Cells. 2022;11:3273.
Funding
The authors would like to thank the São Paulo Research Foundation (FAPESP), grant numbers 2022/02456-0, 2023/07417-6, 2022/02076-3. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES), Finance Code 001. G.M. received a Productivity Fellowship from the Brazilian National Council of Technological and Scientific Development (CNPq 306060/2022-1).
Author information
Authors and Affiliations
Contributions
GM and MGF conceived the subject of the review. MGF, CS and WHR performed the writing and structure of the text. MGF and WHR designed the figures. CS constructed the table. All authors revised, edited and approved the final version of the manuscript, as well as its submission. GM provided financial support.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fontes, M.G., Silva, C., Roldán, W.H. et al. Exploring the potential of asparagine restriction in solid cancer treatment: recent discoveries, therapeutic implications, and challenges. Med Oncol 41, 176 (2024). https://doi.org/10.1007/s12032-024-02424-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-024-02424-3