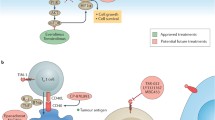
Abstract
In the United States, the estimated number of new cases of renal cell carcinoma (RCC) is approximately 65,000 case with about 15,000 deaths in the year of 2018 (Siegel et al. in CA Cancer J Clin 68(1):7, 2018). RCC as an immunogenic malignancy is supported by many theories and facts which include tumor richness of lymphocytes infiltrate, the occurrence of spontaneous tumor regression, and the proved effect of traditional immunotherapy (Finke et al. in J Immunother 11(1):1–11, 1992), all these factors support the potential therapeutic effect of the novel immunotherapeutic agents in RCC. Historically, complete tumor regression in metastatic RCC is achievable in a minority of patients through traditional immunotherapies such as high-dose interleukin-2 (IL-2) (Fyfe et al. in J Clin Oncol 13(3):688, 1995) and interferon-alfa (IFNa) (Negrier et al. in N Engl J Med 338(18):1272, 1998); however due to the significant rate of toxicities and low efficacy; accordingly the targeted therapy with tyrosine kinase inhibitors (TKIs) and vascular endothelial growth factor-antibodies (VEGF) became the standard and prevalent treatment approach for advanced RCC both in front and subsequent lines of therapy (Escudier et al. in Ann Oncol. 25(Suppl 3):iii49–iii56, 2014). A new avenue of immunotherapy utilizing novel strategy to block immune checkpoints has emerged in a new era for RCC treatment (Ascierto et al. in J Transl Med 12:291, 2014). Results from clinical trials are encouraging in both front-line and second-line settings, in a phase III trial (CheckMate 025) nivolumab compared to everolimus improved overall survival in previously treated metastatic RCC who had progressed on prior treatment with targeting agents (Motzer et al. in N Engl J Med 373:1803, 2015). CheckMate 214, a phase III trial, demonstrated superior overall survival and objective response with combined checkpoint inhibitors compared to sunitinib in Treatment-Naïve Advanced RCC among intermediate- and poor-risk group (Motzer et al. in N Engl J Med. 378(14):1277–1290, 2018). In this review, we discuss the systemic Immunotherapy with checkpoint inhibitors that have been approved or are currently being investigated in RCC, clinical experience with these agents, and its future development.
Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7.
Finke JH, Rayman P, Edinger M, et al. Characterization of a human renal cell carcinoma specific cytotoxic CD8 T cell line. J Immunother. 1992;11(1):1–11.
Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13(3):688.
Negrier S, Escudier B, Lasset C, Douillard JY, Savary J, Chevreau C, Ravaud A, Mercatello A, Peny J, Mousseau M, Philip T, Tursz T. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Français d’Immunothérapie. N Engl J Med. 1998;338(18):1272.
Escudier B, Porta C, Schmidinger M, Algaba F, Patard J, Khoo V, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii49–iii56. https://doi.org/10.1093/annonc/mdu259.
Ascierto P, Addeo R, Cartenì G, Daniele B, De Laurentis M, Ianniello, et al. The role of immunotherapy in solid tumors: report from the Campania society of oncology immunotherapy (SCITO) meeting, Naples 2014. J Transl Med. 2014;12:291.
Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803.
Escudier B, Motzer RJ, Sharma P, et al. Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in CheckMate 025. Eur Urol. 2017;72:368–76.
Escudier B, Sharma P, McDermott DF, et al. CheckMate 025 randomized phase 3 study: outcomes by key baseline factors and prior therapy for nivolumab versus everolimus in advanced renal cell carcinoma. Eur Urol. 2017;72:962–71.
Motzer RJ, Powles T, Atkins MB, et al. IMmotion151: a randomized phase III study of atezolizumab plus bevacizumab versus sunitinib in untreated metastatic renal cell carcinoma. 2018 Genitourinary Cancers Symposium. Abstract 578. Presented February 10, 2018.
Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab + ipilimumab vs sunitinib for treatment-naïve advanced or metastatic renal cell carcinoma (RCC): results from CheckMate 214, including overall survival by subgroups (Abstract O38). Society for Immunotherapy of Cancer 2017 meeting, 2017.
Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients (pts) with advanced renal cell carcinoma (aRCC): preliminary safety and efficacy results. Ann Oncol 2016;6:266 (Abstract 773PD).
Powles T, Albiges L, Staehler M, et al. Updated European association of urology guidelines recommendations for the treatment of first-line metastatic clear cell renal cancer. Eur Urol. 2017;73:311–5.
Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90.
Escudier B, Tannir N, McDermott D, et al. Efficacy and safety of nivolumab + ipilimumab (N + I) v sunitinib (S) for treatment-naïve advanced or metastatic renal cell carcinoma (mRCC), including IMDC risk and PD-L1 expression subgroups (abstract LBA5). Presented at the 2017 European Society of Medical Oncology meeting, 2017.
ClinicalTrials.gov. 2017. Identifier NCT03141177, a study of nivolumab combined with cabozantinib compared to sunitinib in previously untreated advanced or metastatic renal cell carcinoma (CheckMate 9ER), May 4, 2017, [about 4 screens]. Bethesda: National Library of Medicine (US). https://clinicaltrials.gov/ct2/show/NCT03141177?term=checkmate+9ER&cond=Renal+Cell+Carcinoma&rank=1. Accessed 9 April 2018.
Motzer RJ, Powles T, Atkins MD, et al. IMmotion 151: a randomized phase III study of atezolizumab plus bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma (mRCC) abstract 578. Presented on 10 February 2018, Genitourinary Cancer Symposium, San Francisco, USA, 2018.
Atkins MB, Gupta S, Choueiri TK, et al. Phase Ib dose-finding study of axitinib plus pembrolizumab in treatment-naïve patients with advanced renal cell carcinoma. J Immunother Cancer. 2015;3(Suppl 2):P353. https://doi.org/10.1186/2051-1426-3-S2-P353.
Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients (pts) with advanced renal cell carcinoma (aRCC): preliminary safety and efficacy results. Ann Oncol. 2016;27(Suppl 6):773PD. https://doi.org/10.1093/annonc/mdw373.01.
Robert JM, Grünwald V, Hutson TE, et al. A phase III trial to compare efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab vs sunitinib alone in first-line treatment of patients (Pts) with metastatic renal cell carcinoma (RCC). J Clin Oncol. 2017;35(15_suppl): TPS4595–TPS4595.
Choueiri TK, Rini BI, Larkin JMG, et al. Avelumab plus axitinib vs sunitinib as first-line treatment of advanced renal cell carcinoma: Phase 3 study (JAVELIN Renal 101). J Clin Oncol. 2017;35(15_suppl): TPS4594–TPS4594.
Author information
Authors and Affiliations
Contributions
All authors equally contributed to this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
No animal or human were involved in this study. It is a review article. Institutional Review Board is not required.
Rights and permissions
About this article
Cite this article
Alsharedi, M., Katz, H. Check point inhibitors a new era in renal cell carcinoma treatment. Med Oncol 35, 85 (2018). https://doi.org/10.1007/s12032-018-1147-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-018-1147-y