Abstract
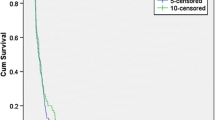
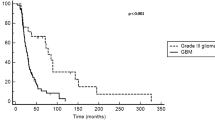
The aim of this study was to evaluate the efficiency and safety of single-agent bevacizumab therapy for recurrent glioblastoma multiforme (GBM). We identified patients with histologically confirmed glioblastoma and World Health Organization Grade III glioma who were previously treated with temozolomide plus radiotherapy and received 10 mg/kg bevacizumab intravenous infusion every 2 weeks until disease progression for recurrent disease. A total 24 patients included to this study. Twenty-two patients had GBM, and two patients had WHO grade III glioma. No complete response was observed, five patients (20.8 %) had partial response, nine patients (37.5 %) had stable diseases, and ten patients (41.7 %) had progressive diseases. The overall response rate was 20.8 %. The 6-month PFS rate (PFS6) and median PFS were determined as 37.5 % and 4.1 months, respectively. Median OS was 6.4 months. Performance status of 17 (70.8 %) patients was improved following bevacizumab regimen. Univariate analysis showed that improvement in performance status (IPS) following bevacizumab therapy was a significant predictor of both PFS (p < 0.001) and OS (p < 0.020). Bevacizumab-related adverse effects were observed in 13 (54.1 %) patients. Grade 3–4 toxicity was observed in 4 (16.6 %) patients. Therapy interruptions were experienced in two patients due to adverse effects. Single-agent bevacizumab is an effective and safe treatment alternative in recurrent GBM. IPS following bevacizumab therapy was a significant predictor of both PFS and OS.


Similar content being viewed by others
References
Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16(8):2443–9.
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83(5):588–93.
Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma—Are we there yet? Neuro-Oncology. 2013;15(1):4–27.
Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76.
Godard S, Getz G, Delorenzi M, Farmer P, Kobayashi H, Desbaillets I, et al. Classification of human astrocytic gliomas on the basis of gene expression: a correlated group of genes with angiogenic activity emerges as a strong predictor of subtypes. Cancer Res. 2003;63(20):6613–25.
Jain HV, Nor JE, Jackson TL. Modeling the VEGF-Bcl-2-CXCL8 pathway in intratumoral angiogenesis. Bull Math Biol. 2008;70(1):89–117.
Fang Y, Qu X, Cheng B, Chen Y, Wang Z, Chen F, et al. The efficacy and safety of bevacizumab combined with chemotherapy in treatment of HER2-negative metastatic breast cancer: a meta-analysis based on published phase III trials. Tumour Biol. 2014. doi:10.1007/s13277-014-2799-7.
Lange A, Prenzler A, Frank M, Golpon H, Welte T, von der Schulenburg JM. A systematic review of the cost-effectiveness of targeted therapies for metastatic non-small cell lung cancer (NSCLC). BMC Pulm Med. 2014;14(1):192.
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–5.
Chamberlain MC, Johnston SK. Salvage therapy with single agent bevacizumab for recurrent glioblastoma. J Neurooncol. 2010;96(2):259–69.
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–40.
Cirillo M, Venturini M, Ciccarelli L, Coati F, Bortolami O, Verlato G. Clinician versus nurse symptom reporting using the National Cancer Institute—common terminology criteria for adverse events during chemotherapy: results of a comparison based on patient’s self-reported questionnaire. Ann Oncol. 2009;20(12):1929–35.
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–72.
Jansen M, de Witt Hamer PC, Witmer AN, Troost D, van Noorden CJ. Current perspectives on antiangiogenesis strategies in the treatment of malignant gliomas. Brain Res Brain Res Rev. 2004;45(3):143–63.
Huang H, Held-Feindt J, Buhl R, Mehdorn HM, Mentlein R. Expression of VEGF and its receptors in different brain tumors. Neurol Res. 2005;27(4):371–7.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62.
Cloughesy T, Prados M, Wen PY. A phase II, randomized, noncomparative clinical trial of bevacizumab alone or in combination with irinotecan prolongs 6-month PFS in recurrent, treatment-refractory glioblastoma [abstract]. J Clin Oncol. 2008;26:2010b.
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–9.
Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66(8):1258–60.
Nagane M, Nishikawa R, Narita Y, Kobayashi H, Takano S, Shinoura N, et al. Phase II study of single-agent bevacizumab in Japanese patients with recurrent malignant glioma. Jpn J Clin Oncol. 2012;42(10):887–95.
Nghiemphu PL, Liu W, Lee Y, Than T, Graham C, Lai A, et al. Bevacizumab and chemotherapy for recurrent glioblastoma: a single-institution experience. Neurology. 2009;72(14):1217–22.
Francesconi AB, Dupre S, Matos M, Martin D, Hughes BG, Wyld DK, et al. Carboplatin and etoposide combined with bevacizumab for the treatment of recurrent glioblastoma multiforme. J Clin Neurosci. 2010;17(8):970–4.
Hasselbalch B, Lassen U, Hansen S, Holmberg M, Sorensen M, Kosteljanetz M, et al. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a phase II trial. Neuro-Oncology. 2010;12(5):508–16.
Sathornsumetee S, Desjardins A, Vredenburgh JJ, McLendon RE, Marcello J, Herndon JE, et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro-Oncology. 2010;12(12):1300–10.
Verhoeff JJ, Lavini C, van Linde ME, Stalpers LJ, Majoie CB, Reijneveld JC, et al. Bevacizumab and dose-intense temozolomide in recurrent high-grade glioma. Ann Oncol. 2010;21(8):1723–7.
Desjardins A, Reardon DA, Coan A, Marcello J, Herndon JE 2nd, Bailey L, et al. Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer. 2012;118(5):1302–12.
Reardon DA, Desjardins A, Peters KB, Gururangan S, Sampson JH, McLendon RE, et al. Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naive, recurrent glioblastoma. J Neurooncol. 2012;107(1):155–64.
Raizer JJ, Grimm S, Chamberlain MC, Nicholas MK, Chandler JP, Muro K, et al. A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer. 2010;116(22):5297–305.
Zhang G, Huang S, Wang Z. A meta-analysis of bevacizumab alone and in combination with irinotecan in the treatment of patients with recurrent glioblastoma multiforme. J Clin Neurosci. 2012;19(12):1636–40.
Conflict and interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hacibekiroglu, I., Kodaz, H., Erdogan, B. et al. Single-agent bevacizumab is an effective treatment in recurrent glioblastoma. Med Oncol 32, 12 (2015). https://doi.org/10.1007/s12032-014-0460-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0460-3