Abstract
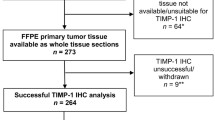
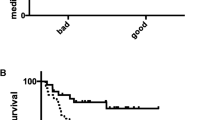
For breast cancer patients with lymph node metastasis, paclitaxel is the first-line chemotherapy drug. Clinical studies showed that some patients with breast cancer were insensitive to paclitaxel, which led to chemotherapy failure. Today, no validated markers exist for the prediction of chemotherapy sensitivity in this patient group. Tissue inhibitor of metalloproteinases-1 (TIMP-1) has been shown to protect against apoptosis. Epidemiological studies have also associated elevated tumor tissue TIMP-1 levels with a poor response to cyclophosphamide/methotrexate/5-fluorouracil and anthracycline-based chemotherapy. Additionally, our previous study proved that TIMP-1 significantly decreased the sensitivity of breast cancer cells to paclitaxel-induced apoptosis by enhancing degradation of cyclin B1. These data imply that TIMP-1 may be a useful predictive biomarker for chemotherapy resistance. In this retrospective study, we investigated the association between expression levels of TIMP-1 protein in the primary tumor and objective response to paclitaxel-based chemotherapy in 99 patients with breast cancer. With Kaplan–Meier survival analysis, the patients with high TIMP-1 levels were found to have significantly worse 5-year DFS (71.1 %) than the patients with low levels (88.5 %; P = 0.020). Similarly, the patients with high TIMP-1 levels had significantly worse 5-year OS (78.9 %) than patients with low levels (96.7 %; P = 0.004). In Cox’s univariate and multivariate analyses, TIMP-1 was prognostic for both DFS and OS. Our data showed that elevated tumor tissue TIMP-1 levels were significantly associated with a poor response to paclitaxel-based chemotherapy, and TIMP-1 might be a potential biomarker for predicting response of breast cancer patients to paclitaxel-based chemotherapy.


Similar content being viewed by others
References
Han W, et al. Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer. 2004;4:82.
Esteva FJ, Hortobagyi GN. Prognostic molecular markers in early breast cancer. Breast Cancer Res. 2004;6(3):109–18.
Verma RP, Hansch C. Taxane analogues against breast cancer: a quantitative structure-activity relationship study. Chem Med Chem. 2008;3(4):642–52.
Robinson WR, Davis N, Rogers AS. Paclitaxel maintenance chemotherapy following intraperitoneal chemotherapy for ovarian cancer. Int J Gynecol Cancer. 2008;18(5):891–5.
Choong NW, et al. Phase I study of induction chemotherapy and concomitant chemoradiotherapy with irinotecan, carboplatin, and paclitaxel for stage III non-small cell lung cancer. J Thorac Oncol. 2008;3(1):59–67.
Agarwala SS, et al. Long-term outcomes with concurrent carboplatin, paclitaxel and radiation therapy for locally advanced, inoperable head and neck cancer. Ann Oncol. 2007;18(7):1224–9.
Hortobagyi GN, Holmes FA. Single-agent paclitaxel for the treatment of breast cancer: an overview. Semin Oncol. 1996;23(1 Suppl 1):4–9.
Bergh J, et al. A systematic overview of chemotherapy effects in breast cancer. Acta Oncol. 2001;40(2–3):253–81.
Bast RC Jr, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19(6):1865–78.
Jordan MA. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem Anticancer Agents. 2002;2(1):1–17.
Abal M, Andreu JM, Barasoain I. Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr Cancer Drug Targets. 2003;3(3):193–203.
Pathan N, et al. Microtubule-targeting drugs induce Bcl-2 phosphorylation and association with Pin1. Neoplasia. 2001;3(1):70–9.
Janssen K, et al. Apaf-1 and caspase-9 deficiency prevents apoptosis in a Bax-controlled pathway and promotes clonogenic survival during paclitaxel treatment. Blood. 2007;110(10):3662–72.
Wurtz SO, et al. Tissue inhibitor of metalloproteinases-1 in breast cancer. Endocr Relat Cancer. 2005;12(2):215–27.
Pesta M, et al. Clinicopathological assessment and quantitative estimation of the matrix metalloproteinases MMP-2 and MMP-7 and the inhibitors TIMP-1 and TIMP-2 in colorectal carcinoma tissue samples. Anticancer Res. 2007;27(4A):1863–7.
Chromek M, et al. Tissue inhibitor of metalloproteinase 1 activates normal human granulocytes, protects them from apoptosis, and blocks their transmigration during inflammation. Infect Immun. 2004;72(1):82–8.
Lee SJ, et al. TIMP-1 inhibits apoptosis in breast carcinoma cells via a pathway involving pertussis toxin-sensitive G protein and c-Src. Biochem Biophys Res Commun. 2003;312(4):1196–201.
Lambert E, et al. Tissue inhibitor of metalloproteinases-1 signalling pathway leading to erythroid cell survival. Biochem J. 2003;372(Pt 3):767–74.
Liu XW, et al. Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells against intrinsic apoptotic cell death via the focal adhesion kinase/phosphatidylinositol 3-kinase and MAPK signaling pathway. J Biol Chem. 2003;278(41):40364–72.
Li G, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 inhibits apoptosis of human breast epithelial cells. Cancer Res. 1999;59(24):6267–75.
Schrohl AS, et al. Primary tumor levels of tissue inhibitor of metalloproteinases-1 are predictive of resistance to chemotherapy in patients with metastatic breast cancer. Clin Cancer Res. 2006;12(23):7054–8.
Wurtz SO, et al. TIMP-1 as a tumor marker in breast cancer—an update. Acta Oncol. 2008;47(4):580–90.
Nakopoulou L, et al. Enhanced mRNA expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) in breast carcinomas is correlated with adverse prognosis. J Pathol. 2002;197(3):307–13.
Schrohl AS, et al. Tumor tissue levels of tissue inhibitor of metalloproteinase-1 as a prognostic marker in primary breast cancer. Clin Cancer Res. 2004;10(7):2289–98.
Wang T, et al. Tissue inhibitor of metalloproteinase-1 protects MCF-7 breast cancer cells from paclitaxel-induced apoptosis by decreasing the stability of cyclin B1. Int J Cancer. 2010;126(2):362–70.
Henderson IC, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21(6):976–83.
Mamounas EP, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23(16):3686–96.
Martin M, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352(22):2302–13.
McGrogan BT, et al. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008;1785(2):96–132.
Acknowledgments
This work was supported by The National Natural Science Foundation of China (Grant 30870978). We have read and have abided by the statement of ethical standards laid down in the 1964 Declaration of Helsinki.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, D., Zha, X., Hu, M. et al. High expression of TIMP-1 in human breast cancer tissues is a predictive of resistance to paclitaxel-based chemotherapy. Med Oncol 29, 3207–3215 (2012). https://doi.org/10.1007/s12032-012-0239-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-012-0239-3