Abstract
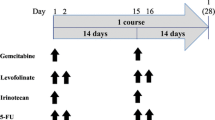
The purpose of this study was to evaluate the efficacy and safety of Zoladex combined with CEF chemotherapy as neoadjuvant therapy in hormone-responsive, premenopausal, operable breast cancer. One hundred and nineteen patients with hormone-responsive, premenopausal, operable breast cancer were enrolled in the study. Zoladex at 3.6 mg was given by subcutaneous injection every 4 weeks for 3 cycles. CEF (cyclophosphamide, 600 mg/m2, epirubicin, 60–90 mg/m2, and fluorouracil 500 mg/m2) was administered every 3 weeks for 4 cycles as neoadjuvant therapy. The primary objective was a pathologic complete response (PCR) rate in the breast. Thirty-one patients (26.1%) achieved a clinical complete response, and 76 patients (63.9%) achieved a clinical partial response; the clinical response rate was 90.0%. Fourteen patients (11.8%) achieved a pathologic complete response (T0/Tis, N0), and 20 patients (16.8%) achieved a pathologic complete response (T0/Tis, Nx). When stratified by the clinical lymph node status, the clinical partial response rate in the clinical lymph node negative group was significantly higher than in the clinical lymph node positive group (P = 0.02). When stratified by hormonal status, the clinical partial response rate in the ER and PR + group was significantly better than the ER or PR− group (P = 0.0471). There were no treatment-related deaths and no grades 3 or 4 toxicity. The most common adverse event was nausea (grade 1 65.5%, grade 2 18.5%), vomiting (grade 1 58.8%, grade 2 13.4%), and alopecia (grade 1 45.4%, grade 2 54.6%). Zoladex combined with CEF chemotherapy was effective as neoadjuvant therapy in hormone-responsive, premenopausal breast cancer. This regimen was particularly effective in the clinical lymph node negative group and in the ER/PR double positive group. (ClinicalTrials.gov number, NCT00488722).
Similar content being viewed by others
References
McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ. 2000;321:624–8.
Yang L, Parkin DM, Ferlay J, et al. Estimates of cancer incidence in China for 2000 and projections for 2005. Cancer Epidemiol Biomarkers Prev. 2005;14:243–50.
Gradishar W. Landmark trials in endocrine adjuvant therapy for breast carcinoma. Cancer. 2006;106:975–81.
Rody A, Loibl S, von Minckwitz G, et al. Use of goserelin in the treatment of breast cancer. Expert Rev Anticancer Ther. 2005;5:591–604.
Jonat W, Kaufmann M, Sauerbrei W, et al. Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: the Zoladex early breast cancer research association study. J Clin Oncol. 2002;20:4628–35.
Fogelman I, on behalf of the ZEBRA Trialists’ Group. Assessment of bone mineral density in pre-/perimenopausal women treated with Zoladex TM versus CMF adjuvant terapy for management of nodepositive, early breast cancer: results from the ZEBRA study. Breast. 2001;10(Suppl 1): S31, Abstr P59.
de Haes H, Olschewswki M, Schumaker M, et al. Early benefits in quality of life (Qol) observed in Zoladex TM—treated versus CMF-treated pre-/perimenopaysal patients with node-positive early breast cancer. Proc ASCO. 2001;20:35a,Abstr138.
Name M, Jonat W, Kaufman M, et al. Survial data from the ZEBRA study. Ann Oncol. 2002;13(suppl 5):38, Abstr 135P.
Castiglinoe-Gertsch M, O’Neill A, Gelber RD, et al. Is the addition of adjuvant chemotherapy always necessary in node negative(N-) pre/perimenopaysal breast cancer patients (pts) who receive goserelin? First results of IBCSG trial VIII. Proc ASCO. 2002;21:38a, Abstr 149.
Castiglione-Gertsch M, Gelber RD, O’Neill A, et al. Systemic adjuvant treatment for premenopausal node-negative breast cancer. Eur J Cancer. 2000;36:549–50.
Jakesz R, Hausmaninger H, Kubista E, et al. Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer–Austrian breast and colorectal cancer study group trial 5. J Clin Oncol. 2002;20:4621–7.
Boccardo F, Rubagotti A, Amoroso D, et al. Cyclophosphamide, methotrexate, and fluorouracil versus tamoxifen plus ovarian suppression as adjuvant treatment of estrogen receptor-positive pre-/perimenopausal breast cancer patients: results of the italian breast cancer adjuvant study group 02 randomized trial. J Clin Oncol. 2000;18:2718–27.
Boccardo F, Rubagotti A, Amoroso D, et al. CMF versus tamoxifen (TAM) plus ovarian suppres sion (OS) as adjuvant treatment of ER-positive (ER+) pre/perimenopausal breast cancer (BCA) patients(pts). Breast. 2001;10(Suppl 1):S32, Abstr P62.
Kuter I. Breast cancer highlights. Oncologist. 1999;4:299–308.
Bianco AR, Costanzo R, Di Lorenzo G, et al. The Mam-1 GOCSI trial: a randomised trial with factorial design of chemo-endocrine adjuvant treatment in node-positive (N+) early breast cancer (ebc). Proc ASCO. 2001;20:27a, Abstr 104.
Liu ZY, Zhang J. Comparison clinical effect of TE versus CEF regimens as neoadjuvant chemotherapy in the treatment of breast cancer. Chin J Breast Cancer. 2008;2:30–9.
Livasy CA, Karaca G, Nanda R, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19:264–71.
Rodríguez-Pinilla SM, Sarrió D, Honrado E, Moreno-Bueno G, Hardisson D, Calero F, Benítez J, Palacios J. Vimentin and laminin expression is associated with basal-like phenotype in both sporadic and BRCA1-associated breast carcinomas. J Clin Pathol. 2007;60:1006–12.
Rakha EA, El-Rehim DA, Paish C, et al. Basal phenotype identifies a poor prognostic subgroup of breast cancer of clinical importance. Eur J Cancer. 2006;42:3149–56.
Dalerba P, Clarke MF. Cancer stem cells and tumor metastasis: first steps into uncharted territory. Cell Stem Cell. 2007;1:241–2.
Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–61.
Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–34.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Sheng Zhang and Chongjian Zhang contributed equally to this article.
Rights and permissions
About this article
Cite this article
Zhang, S., Zhang, C., Liu, J. et al. A Phase II trial of Zoladex combined with CEF chemotherapy as neoadjuvant therapy in premenopausal women with hormone-responsive, operable breast cancer. Med Oncol 29, 479–485 (2012). https://doi.org/10.1007/s12032-011-9883-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-9883-2