Abstract
Vanishing white matter disease (VWM) is a rare autosomal recessive leukodystrophy caused by a mutation in any of the five gene encoding subunits of the translation initiation factors eIF2B1 to eIF2B5. Whole-exome sequencing was performed on a 7-year-old boy with prenatal symptoms, including intrauterine-growth retardation, decreased movements, and oligohydramnios as well as mild intellectual disability, optic atrophy, macrocephaly, mild ataxia, and white matter lesions after birth. Analysis of WES data revealed a homozygous missense variant, c.C590T (p.Thr197Met) in the EIF2B3 gene (NM_0203650). The candidate variant was confirmed by Sanger sequencing and found to co-segregate with disease in family members. Pathogenicity analysis, 3D protein modeling, and stability assessment showed the deleterious effects of this nucleotide change. Previous studies suggest a direct relationship between the onset of symptoms and the progression rate and severity of the disease. All described cases of EIF2B deficiency with antenatal-onset led prenatal death; if they were born, they experienced clinical exacerbation, seizure, severe encephalopathy, and consequent infantile death (< 1 year). The patient of this study had never had seizure, which could be a potential explanation for the observed mild clinical picture, chronic state, and long-term survival until the age of seven. This study reported the first VWM due to EIF2B gene deficiency with antenatal-onset but mild symptoms and long-term survival. The result of this study showed that stressor factors, particularly seizure, could have a substantial role in poor prognosis and early neonatal death.



Similar content being viewed by others
Data Availability
Data are available upon reasonable request.
References
Abbink TE, Wisse LE, Wang X, Proud CG (2018) Role of Eukaryotic initiation factor eIF2B in vanishing white matter disease. The Oxford Handbook of Neuronal Protein Synthesis
Accogli A, Brais B, Tampieri D, La Piana R (2019) Long-standing psychiatric features as the only clinical presentation of vanishing white matter disease. J Neuropsychiatry Clin Neurosci 31(3):276–279
Alsalem A, Shaheen R, Alkuraya FS (2012) Vanishing white matter disease caused by EIF2B2 mutation with the presentation of an adrenoleukodystrophy phenotype. Gene 496(2):141–143
Bugiani M, Boor I, Powers JM, Scheper GC, van der Knaap MS (2010) Leukoencephalopathy with vanishing white matter: a review. J Neuropathol Exp Neurol 69(10):987–996
Bugiani M, van der Knaap MS (2017) Childhood white matter disorders: much more than just diseases of myelin. Springer
Fontenelle LMDC, Scheper GC, Brandão L, Van der Knaap MS (2008) Atypical presentation of vanishing white matter disease. Arq Neuropsiquiatr 66(3A):549–551
Güngör G, Güngör O, Çakmaklı S, Genç HM, İnce H, Yeşil G et al (2020) Vanishing white matter disease with different faces. Childs Nerv Syst 36(2):353–361
Hamilton EM, van der Lei HD, Vermeulen G, Gerver JA, Lourenço CM, Naidu S et al (2018) Natural history of vanishing white matter. Ann Neurol 84(2):274–288
Ho CS, Mangelsdorf S, Walterfang M (2020) The disappearance of white matter in an adult-onset disease: a case report. BMC Psychiatry 20(1):1–4
Kevelam SH, Steenweg ME, Srivastava S, Helman G, Naidu S, Schiffmann R et al (2016) Update on leukodystrophies: a historical perspective and adapted definition. Neuropediatrics 47(06):349–354
Köhler W, Curiel J, Vanderver A (2018) Adulthood leukodystrophies. Nature Reviews. Neurology 14(2):94
Leegwater PA, Vermeulen G, Könst AA, Naidu S, Mulders J, Visser A et al (2001) Subunits of the translation initiation factor eIF2B are mutant in leukoencephalopathy with vanishing white matter. Nat Genet 29(4):383–388
Lynch DS, de Paiva ARB, Zhang WJ, Bugiardini E, Freua F, Tavares Lucato L et al (2017) Clinical and genetic characterization of leukoencephalopathies in adults. Brain 140(5):1204–1211
Pavitt GD, Proud CG (2009) Protein synthesis and its control in neuronal cells with a focus on vanishing white matter disease. Biochem Soc Trans 37(6):1298–1310
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–423
Scali O, Di Perri C, Federico A (2006) The spectrum of mutations for the diagnosis of vanishing white matter disease. Neurol Sci 27(4):271–277
Scheper GC, Proud CG, van der Knaap MS (2006) Defective translation initiation causes vanishing of cerebral white matter. Trends Mol Med 12(4):159–166
Schiffmann R, Moller JR, Trapp BD, Shih HHL, Farrer RG, Katz DA et al (1994) Childhood ataxia with diffuse central nervous system hypomyelination. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 35(3):331–340
Song H, Haeri S, Vogel H, Van Der Knaap M, Van Haren K (2017) Postmortem whole exome sequencing identifies novel EIF2B3 mutation with prenatal phenotype in 2 siblings. J Child Neurol 32(10):867–870
Takano K, Tsuyusaki Y, Sato M, Takagi M, Anzai R, Okuda M et al (2015) A Japanese girl with an early-infantile onset vanishing white matter disease resembling Cree leukoencephalopathy. Brain Dev 37(6):638–642
Trimouille A, Marguet F, Sauvestre F, Lasseaux E, Pelluard F, Martin-Négrier ML et al (2020) Foetal onset of EIF2B related disorder in two siblings: cerebellar hypoplasia with absent Bergmann glia and severe hypomyelination. Acta Neuropathol Commun 8:1–7
Van der Knaap M, Bugiani M, Boor I, Proud C, Scheper G (2010) Vanishing white matter. Neurogenetics. (Part 28)
van der Knaap MS, Bugiani M (2017) Leukodystrophies: a proposed classification system based on pathological changes and pathogenetic mechanisms. Acta Neuropathol 134(3):351–382
Van Der Knaap MS, Leegwater PA, Könst AA, Visser A, Naidu S, Oudejans CB et al (2002) Mutations in each of the five subunits of translation initiation factor eIF2B can cause leukoencephalopathy with vanishing white matter. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 51(2):264–270
van der Knaap MS, Pronk JC, Scheper GC (2006) Vanishing white matter disease. Lancet Neurol 5(5):413–423
van der Knaap MS, Schiffmann R, Mochel F, Wolf NI (2019) Diagnosis, prognosis, and treatment of leukodystrophies. Lancet Neurol 18(10):962–972
Van Der Knaap MS, Van Berkel CG, Herms J, Van Coster R, Baethmann M, Naidu S et al (2003) eIF2B-related disorders: antenatal onset and involvement of multiple organs. Am J Hum Genet 73(5):1199–1207
Wei C, Qin Q, Chen F, Zhou A, Wang F, Zuo X et al (2019) Adult-onset vanishing white matter disease with the EIF2B2 gene mutation presenting as menometrorrhagia. BMC Neurol 19(1):203
Woody AL, Hsieh DT, McIver HK, Thomas LP, Rohena L (2015) Infantile onset vanishing white matter disease associated with a novel EIF2B5 variant, remarkably long life span, severe epilepsy, and hypopituitarism. Am J Med Genet A 167(4):826–830
Wu Y, Pan Y, Du L, Wang J, Gu Q, Gao Z et al (2009) Identification of novel EIF2B mutations in Chinese patients with vanishing white matter disease. J Hum Genet 54(2):74–77
Acknowledgements
We would like to express our special thanks to our patients, their family, medical genetics lab GENEAZMA, and Isfahan University of Medical Science for their collaboration.
Funding
The project was supported by deputies of research of Isfahan University of Medical Sciences [194068].
Author information
Authors and Affiliations
Contributions
MK contributed in data analysis and interpretation, drafted and revised the manuscript. EK contributed in manuscripts writing and laboratory works. MR contributed in data collection and laboratory works. AH: contributed in data analysis and interpretation. OI and OY supervised the clinical evaluation. MR contributed in clinical evaluation. VY contributed in data collection and manuscript revision. MAK designed and supervised the study. All of the authors read and approved the final manuscript to be published and agreed to be responsible for the accuracy of the data and details.
Corresponding author
Ethics declarations
Ethics Approval
The study was approved by the Ethical Committee of the Isfahan University of Medical Science (IR.MUI.MED.REC.1398.186).
Consent to Participate
Written informed consent was obtained from the legal guardian of patients (parents).
Consent for Publication
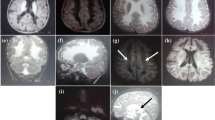
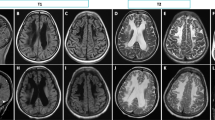
Written informed consent for publication of clinical details and images (Fig. 2 and Supplementary Fig. 1) was obtained from the patient legal guardian.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khorrami, M., Khorram, E., Yaghini, O. et al. Identification of a Missense Variant in the EIF2B3 Gene Causing Vanishing White Matter Disease with Antenatal-Onset but Mild Symptoms and Long-Term Survival. J Mol Neurosci 71, 2405–2414 (2021). https://doi.org/10.1007/s12031-021-01810-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-021-01810-0