Abstract
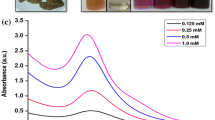
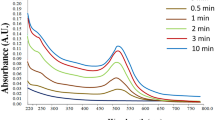
Addition of microwave-exposed aqueous extracellular anti-malignant guava (Psidium guajava) leaf extract to the aqueous gold chloride solution yielded stable polyshaped gold nanoparticles of high composition. Microwave-assisted route selected for the preparation of aqueous guava leaf extract was to suppress the enzymatic action. The formation of nanoparticles was understood from the UV–visible and X-ray diffraction studies. The size and shape analysis was done using field emission scanning electron microscopy, transmission electron microscopy, and atomic force microscopy. Zeta potential experiment shows that the bio-functionalized gold nanoparticles colloidal solution obtained as above will maintain its stability even after 30 weeks of storage. It is observed that the flavonoids which are separated during microwave heating of extracellular solution of the guava leaves are responsible for the biosynthesis of gold nanoparticles.











Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Chen C, Sandra CM, Oyelere AK. Gold nanoparticles: From nanomedicine to nanosensing. Nanotechnology, Science and Applications. 2008;1:45.
Salata OV. Application of nanoparticles in biology and medicine. J Nanobiotechnology. 2004;2:3.
El-Sayed M. Small is different: shape-, size-, and composition-dependent properties of some colloidal semiconductor nanocrystals. Acc Chem Res. 2004;37(5):326.
Kim F, Connor S, Song H, Kuykendall T, Yang P. Platonic gold nanocrystals. Angew Chem. 2004;116:3759.
Yakimovich NO, Ezhevskii AA, Guseinov DV, Smirnova LA, Gracheva TA, Klychkov KS. Antioxidant properties of gold nanoparticles studied by ESR spectroscopy. Russ Chem Bull. 2008;57(3):520.
Jing-Liang L, Wang L, Xiang-Yang L, Zhang Z, Hong-Chen G, Wei-Min L, et al. In vitro cancer cell imaging and therapy using transferrin-conjugated gold nanoparticles. Cancer Lett. 2009;27(2):319.
Sperling RA, PR Gil PR, Zhang F, Zanella M, Parak WJ. Biological applications of gold nanoparticle. Chem Soc Rev. 2008;37:1896.
Greenfield AS. Biotechnology, the brain and the future. Trends Biotech. 2005;23(1):34.
Kevin B. Shape Matters for Nanoparticles; Technology published by MIT review; 2008.
Gobin MA, Lee MH, Naomi JH, William DJ, Rebekah AD, Jennifer LW. Near- infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 2007;7(7):1929.
Bertussi B, Natoli JY, Commandre M, Rullier JL, Bonneau F, Combis P, et al. Photothermal investigation of the laser-induced modification of a single gold nano-particle in a silica film. Opt Commun. 2005;254(4):299.
Kannan R, Rahing V, Cutler CS, Pandrapragada RK, Katti KK, Kattumuri VJ, et al. Nanocompatible chemistry toward fabrication of target-specific gold nanoparticles. J Am Chem Soc. 2006;128:11342.
Rinaldo P, Matteo G, Maila S. Nanosystems, in inorganic and bio-inorganic chemistry. In: Bertini I, editor. Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO. Oxford, UK: Eolss Publishers 2006.
Jianqiang H, Zhouping H, Jinghong L. Gold nanoparticles with special shapes: controlled synthesis, surface-enhanced Raman scattering, and the application in biodetection. Sensors. 2007;7:3299.
He L, Musick MD, Nicewarner SR, Salinas FG, Benkovic SJ, Natan MJ, et al. Colloidal Au-enhanced surface plasmon resonance for ultrasensitive detection of DNA hybridization. J Am Chem Soc. 2000;122:9071.
Maxwell DJ, Taylor JR, Nie SM. Self-assembled nanoparticle probes for recognition and detection of biomolecules. J Am Chem Soc. 2002;124:9606.
Haynes CA, Nordle W. Globular proteins at solid/liquid interfaces. Coll Surf B: Biointerfaces. 1994;2:517.
Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R, et al. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Coll Surf B: Biointerfaces. 2008;28:313.
Basavaraja S, Balaji SD, Lagashetty A, Rajasab AH, Venkataraman A. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater Res Bull. 2008;43:1164.
Balaji DS, Basavaraja S, Raghunandan D, DB Mahesh D, Venkataraman A. Biosynthesis and stabilization of Au and Au–Ag alloy nanoparticles by fungus, Fusarium semitectum. Sci Technol Adv Mater. 2008;9:035012.
Balaji DS, Basavaraja S, Raghunandan D, Mahesh B, Prabhakar BK, Venkataraman A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Coll Surf B: Biointerfaces. 2009;68(1):88.
Gardea-Torresdey LJ, Gomez E, Peralta-Videa RJ, Parsons JG, Troiani H, Jose-Yacaman M. Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir. 2003;19(4):1357.
Shiv Shankar S, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275(2):496.
Kawakami Y, Nakamura T, Hosokawa T, Suzuki-Yamamoto T, Yamashita H, Kimoto M, et al. Antiproliferative activity of guava leaf extract via inhibition of prostaglandin endoperoxide H synthase isoforms. Prostaglandins, Leukot Essent Fat Acids. 2009;80(5-6):239.
Manosroi J, Dhumtanom P, Manosroi A. Antiproliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006;235:114.
Chen KC, Hsieh CL, Peng CC, Hsieh-Li HM, Chiang HS, Huang KD, et al. Brain derived metastatic prostate cancer DU-145 cells are effectively inhibited in vitro by guava (Psidium gujava L.) leaf extracts. 58(1). Nutr Cancer. 2007;58:93.
Gutierrez RM, Mitchell S, Solaris RV. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2008;117(1):1.
Choi SY, Hwang JH, Park SY, Jin YJ, Ko HC, Moon SW, et al. Fermented guava leaf extract inhibits LPS-induced COX-2 and iNOS expression in mouse macrophage cells by inhibition of transcription factor NF-kB. Phytother Res. 2008;22:1030.
Mulvaney P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir. 1996;12:788.
Robert BB, David JB, Toca-Herrera LJ, Blake AW, Smith DA, Radford SE, et al. Force mode atomic force microscopy as a tool for protein folding studies. Anal Chim Acta. 2003;479(1):87.
Suganya T, Ikegami F, Okonogi S. Antioxidant active principles isolated from Psidium guajava grown in Thailand. Sci Pharm. 2007;75:179.
Begum S, Hassan SI, Ali SN, Siddiqui BS. Chemical constituents from the leaves of Psidium guajava. Nat Prod Res. 2004;18(2):135.
Zeta potential of colloids in water and waste water, ASTM standard D 4187-82, American society for testing and materials, 1985.
Acknowledgments
Financial supports from DST (grant no.SR/S1/PC-10/2005), UGC (D.O. no.F.14-4/2001 (Innov.Policy/ASIST)), and BRNS, DAE (no. 2009/34/BRNS) are acknowledged. We thank Prof. G. U. Kulkarni for fruitful guidance and Selvi Rajan, JNCASR Bangalore for FESEM measurements. We are grateful to Prof. Manohar Badiger (NCL, Pune) for zeta potential measurements. Raghunandan Deshpande thanks Dr. Appala Raju, Principal of HKES College of pharmacy, Gulbarga for encouraging the research program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raghunandan, D., Basavaraja, S., Mahesh, B. et al. Biosynthesis of Stable Polyshaped Gold Nanoparticles from Microwave-Exposed Aqueous Extracellular Anti-malignant Guava (Psidium guajava) Leaf Extract. Nanobiotechnol 5, 34–41 (2009). https://doi.org/10.1007/s12030-009-9030-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12030-009-9030-8