Abstract
Background
Remote ischemic lesions on diffusion-weighted imaging (DWI) occur in one third of patients with intracerebral hemorrhage (ICH) and are associated with worse outcomes. The etiology is unclear and not solely due to blood pressure reduction. We hypothesized that impaired cerebrovascular autoregulation and hypoperfusion below individualized lower limits of autoregulation are associated with the presence of DWI lesions.
Methods
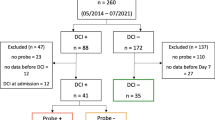
This was a retrospective, single-center study of all primary ICH with intraparenchymal pressure monitoring within 10 days from onset and subsequent magnetic resonance imaging. Pressure reactivity index was calculated as the correlation coefficient between mean arterial pressure and intracranial pressure. Optimal cerebral perfusion pressure (CPPopt) is the cerebral perfusion pressure (CPP) with the lowest corresponding pressure reactivity index. The difference between CPP and CPPopt, time spent below the lower limit of autoregulation (LLA), and time spent above the upper limit of autoregulation (ULA) were calculated by using mean hourly physiologic data. Univariate associations between physiologic parameters and DWI lesions were analyzed by using binary logistic regression.
Results
A total of 505 h of artifact-free data from seven patients without DWI lesions and 479 h from six patients with DWI lesions were analyzed. Patients with DWI lesions had higher intracranial pressure (17.50 vs. 10.92 mm Hg; odds ratio 1.14, confidence interval 1.01–1.29) but no difference in mean arterial pressure or CPP compared with patients without DWI lesions. The presence of DWI lesions was significantly associated with a greater percentage of time spent below the LLA (49.85% vs. 14.70%, odds ratio 5.77, confidence interval 1.88–17.75). No significant association was demonstrated between CPPopt, the difference between CPP and CPPopt, ULA, LLA, or time spent above the ULA between groups.
Conclusions
Blood pressure reduction below the LLA is associated with ischemia after acute ICH. Individualized, autoregulation-informed targets for blood pressure reduction may provide a novel paradigm in acute management of ICH and require further study.



Similar content being viewed by others
References
Murthy SB, Cho SM, Gupta A, Shoamanesh A, Navi BB, Avadhani R, et al. A pooled analysis of diffusion-weighted imaging lesions in patients with acute intracerebral hemorrhage. JAMA Neurol. 2020;77(11):1390–7.
Prabhakaran S, Gupta R, Ouyang B, John S, Temes RE, Mohammad Y, et al. Acute brain infarcts after spontaneous intracerebral hemorrhage. Stroke. 2010;41(1):89–94.
Wu B, Yao X, Lei C, Liu M, Selim MH. Enlarged perivascular spaces and small diffusion-weighted lesions in intracerebral hemorrhage. Neurology. 2015;85(23):2045–52.
Garg RK, Khan J, Dawe RJ, Conners J, John S, Prabhakaran S, et al. The influence of diffusion weighted imaging lesions on outcomes in patients with acute spontaneous intracerebral hemorrhage. Neurocrit Care. 2020;33(2):552–64.
Kidwell CS, Rosand J, Norato G, Dixon S, Worrall BB, James ML, et al. Ischemic lesions, blood pressure dysregulation, and poor outcomes in intracerebral hemorrhage. Neurology. 2017;88(8):782–8.
Shoamanesh A, Cassarly C, Morotti A, Romero JM, Oliveira-Filho J, Schlunk F, et al. Intensive blood pressure lowering and DWI lesions in intracerebral hemorrhage: exploratory analysis of the ATACH-2 randomized trial. Neurocrit Care. 2022;36(1):71–81.
Diedler J, Sykora M, Rupp A, Poli S, Karpel-Massler G, Sakowitz O, et al. Impaired cerebral vasomotor activity in spontaneous intracerebral hemorrhage. Stroke. 2009;40(3):815–9.
Jaeger M, Soehle M, Schuhmann MU, Meixensberger J. Clinical significance of impaired cerebrovascular autoregulation after severe aneurysmal subarachnoid hemorrhage. Stroke. 2012;43(8):2097–101.
Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30(4):733–8.
Gupta VP, Garton ALA, Sisti JA, Christophe BR, Lord AS, Lewis AK, et al. Prognosticating functional outcome following intracerebral hemorrhage: the ICHOP score. World Neurosurg. 2017;101:577–83.
Hemphill J. Claude, Greenberg Steven M., Anderson Craig S., Becker Kyra, Bendok Bernard R., Cushman Mary, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015;46(7):2032–60.
Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults. Stroke. 2007;38(6):2001–23.
Morgenstern LB, Hemphill JC, Anderson C, Becker K, Broderick JP, Connolly ES, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2010;41(9):2108–29.
Liu X, Maurits NM, Aries MJH, Czosnyka M, Ercole A, Donnelly J, et al. Monitoring of optimal cerebral perfusion pressure in traumatic brain injured patients using a multi-window weighting algorithm. J Neurotrauma. 2017;34(22):3081–8.
Aries MJH, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40(8):2456–63.
Sorrentino E, Diedler J, Kasprowicz M, Budohoski KP, Haubrich C, Smielewski P, et al. Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care. 2012;16(2):258–66.
Tas J, Beqiri E, van Kaam RC, Czosnyka M, Donnelly J, Haeren RH, et al. Targeting autoregulation-guided cerebral perfusion pressure after traumatic brain injury (COGiTATE): a feasibility randomized controlled clinical trial. J Neurotrauma. 2021;38(20):2790–800.
Ma H, Guo ZN, Liu J, Xing Y, Zhao R, Yang Y. Temporal course of dynamic cerebral autoregulation in patients with intracerebral hemorrhage. Stroke. 2016;47(3):674–81.
Murthy SB, Diaz I, Wu X, Merkler AE, Iadecola C, Safford MM, et al. Risk of arterial ischemic events after intracerebral hemorrhage. Stroke. 2020;51(1):137–42.
Murthy SB, Zhang C, Gupta A, Cho SM, Lara LR, Avadhani R, et al. Diffusion-weighted imaging lesions after intracerebral hemorrhage and risk of stroke: a MISTIE III and ATACH-2 analysis. Stroke. 2021;52(2):595–602.
Menon RS, Burgess RE, Wing JJ, Gibbons MC, Shara NM, Fernandez S, et al. Predictors of highly prevalent brain ischemia in intracerebral hemorrhage. Ann Neurol. 2012;71(2):199–205.
Gioia LC, Kate M, Choi V, Sivakumar L, Jeerakathil T, Kosior J, et al. Ischemia in intracerebral hemorrhage is associated with leukoaraiosis and hematoma volume, not blood pressure reduction. Stroke. 2015;46(6):1541–7.
Garg RK, Liebling SM, Maas MB, Nemeth AJ, Russell EJ, Naidech AM. Blood pressure reduction, decreased diffusion on mri, and outcomes after intracerebral hemorrhage. Stroke J Cereb Circ. 2012;43(1):67–71.
Qureshi AI, Huang W, Lobanova I, Barsan WG, Hanley DF, Hsu CY, et al. Outcomes of intensive systolic blood pressure reduction in patients with intracerebral hemorrhage and excessively high initial systolic blood pressure. JAMA Neurol. 2020;77(11):1–11.
Buletko AB, Thacker T, Cho SM, Mathew J, Thompson NR, Organek N, et al. Cerebral ischemia and deterioration with lower blood pressure target in intracerebral hemorrhage. Neurology. 2018;91(11):e1058–66.
Strandgaard S. Autoregulation of cerebral blood flow in hypertensive patients: the modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug-induced hypotension. Circulation. 1976;53(4):720–7.
Reinhard M, Lorenz L, Sommerlade L, Allignol A, Urbach H, Weiller C, et al. Impaired dynamic cerebral autoregulation in patients with cerebral amyloid angiopathy. Brain Res. 2019;1717:60–5.
Boulanger M, Schneckenburger R, Join-Lambert C, Werring DJ, Wilson D, Hodel J, et al. Diffusion-weighted imaging hyperintensities in subtypes of acute intracerebral hemorrhage. Stroke. 2018;STROKEAHA118021407.
Tsivgoulis G, Katsanos AH, Butcher KS, Boviatsis E, Triantafyllou N, Rizos I, et al. Intensive blood pressure reduction in acute intracerebral hemorrhage: a meta-analysis. Neurology. 2014;83(17):1523–9.
Kuwata N, Kuroda K, Funayama M, Sato N, Kubo N, Ogawa A. Dysautoregulation in patients with hypertensive intracerebral hemorrhage. A SPECT study. Neurosurg Rev. 1995;18(4):237–45.
Gould B, McCourt R, Asdaghi N, Dowlatshahi D, Jeerakathil T, Kate M, et al. Autoregulation of cerebral blood flow is preserved in primary intracerebral hemorrhage. Stroke. 2013;44(6):1726–8.
Powers WJ, Zazulia AR, Videen TO, Adams RE, Yundt KD, Aiyagari V, et al. Autoregulation of cerebral blood flow surrounding acute (6 to 22 hours) intracerebral hemorrhage. Neurology. 2001;57(1):18–24.
Oeinck M, Neunhoeffer F, Buttler KJ, Meckel S, Schmidt B, Czosnyka M, et al. Dynamic cerebral autoregulation in acute intracerebral hemorrhage. Stroke. 2013;44(10):2722–8.
Reinhard M, Neunhoeffer F, Gerds TA, Niesen WD, Buttler KJ, Timmer J, et al. Secondary decline of cerebral autoregulation is associated with worse outcome after intracerebral hemorrhage. Intensive Care Med. 2010;36(2):264–71.
Ko SB, Choi HA, Parikh G, Helbok R, Schmidt JM, Lee K, et al. Multimodality monitoring for cerebral perfusion pressure optimization in comatose patients with intracerebral hemorrhage. Stroke. 2011;42(11):3087–92.
Diedler J, Santos E, Poli S, Sykora M. Optimal cerebral perfusion pressure in patients with intracerebral hemorrhage: an observational case series. Crit Care Lond Engl. 2014;18(2):R51.
Thomas E, Czosnyka M, Hutchinson P. Calculation of cerebral perfusion pressure in the management of traumatic brain injury: joint position statement by the councils of the Neuroanaesthesia and Critical Care Society of Great Britain and Ireland (NACCS) and the Society of British Neurological Surgeons (SBNS). Br J Anaesth. 2015;115(4):487–8.
Acknowledgements
We would like to thank the physicians and nurses of the neurology and neurosurgery department for their support of this project.
Funding
This study received no external funding.
Author information
Authors and Affiliations
Contributions
Mohamed Ridha MD contributed to data analysis, interpretation, and drafting of the manuscript. Murad Megjhani PhD contributed to the analysis and interpretation of data. Daniel Nametz contributed to data acquisition. Soon Bin Kwon PhD contributed to data interpretation and review. Angela Velazquez MD contributed to data acquisition. Shivani Ghoshal MD contributed to critical intellectual review of the manuscript. Sachin Agarwal MD MPH contributed to critical intellectual review of the manuscript. Jan Claassen MD contributed to critical intellectual review of the manuscript. David J. Roh MD contributed to critical intellectual review of the manuscript. E. Sander Connolly Jr MD contributed to critical intellectual review of the manuscript. Soojin Park MD contributed to design conception, data interpretation, and critical intellectual review of the manuscript. The authors approved of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
SP is supported by National Institutes of Health grants R01NS129760-01 and R01NS131606-01. JC is supported by grant funding from R01NS106014-02S1, R21 NS128326-01, R03 NS112760, R01NS106014-02S2. He received consulting fees from Marinus and is a minority shareholder at iCE Neurosystems. The remaining authors declare no conflict of interest.
Ethical Approval/Informed Consent
The study was approved by the institutional review board of Columbia University Medical Center and was performed in accordance with the ethical standards as outlined in the 1964 Declaration of Helsinki and its amendments. Informed consent was obtained from the patient representative.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ridha, M., Megjhani, M., Nametz, D. et al. Suboptimal Cerebral Perfusion is Associated with Ischemia After Intracerebral Hemorrhage. Neurocrit Care (2023). https://doi.org/10.1007/s12028-023-01863-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12028-023-01863-6