Abstract
Background
The management of cerebral venous sinus thrombosis (CVT) is a common problem facing vascular neurologists. American Heart Association/American Stroke Association guidelines suggest the use of heparin followed by vitamin K antagonists (VKAs) for anticoagulation in CVT. In recent years, the evidence base has solidified for the use of non-vitamin K antagonist oral anticoagulants (NOACs) in lower extremity deep vein thrombosis. Because data supporting their use in CVT are limited, with the strongest evidence comprising one randomized controlled trial of dabigatran, we sought to review our experience with NOACs in the treatment of CVT at a tertiary care center to address efficacy and safety.
Methods
We retrospectively reviewed charts of all patients with CVT treated with an NOAC at our tertiary care facility in the years 2011–2019. We collected data on demographics, risk factors for CVT, clinical features at presentation, imaging results, anticoagulation regimen, bleeding complications, and disability at follow-up. We compared disability at follow-up and major hemorrhagic events with age-matched and sex-matched controls treated with VKAs over the same time period and with historical controls.
Results
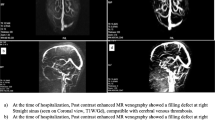
We identified 29 patients with CVT treated with an NOAC, 27 of whom had follow-up within our system. NOACs that were used for treatment included apixaban (20 patients), rivaroxaban (6 patients) and dabigatran (1 patient). NOAC use was associated with stabilization of a clot or partial recanalization in 55.6% of patients and complete recanalization in 14.8% at a median follow-up time of 6 months. The median modified Rankin Score (mRS) at follow-up was 0, with one death. Three patients (11.1%) had major bleeding complications, including two with symptomatic worsening of intracranial hemorrhage. Comparisons of 27 age-matched and sex-matched controls treated with VKAs showed no significant differences in terms of partial recanalization (55.6% vs. 63.0%, p = 0.29), complete recanalization (14.8% vs. 25.9%, p = 0.73), mRS at follow-up (median 0 vs. 0, p = 0.23), or major bleeding (11.1% vs. 11.1%, p > 0.99). Comparisons with the historical International Study on Cerebral Vein and Dural Sinus Thrombosis cohort showed similar functional outcomes: 92.6% of patients treated with NOACs and 88.9% of patients treated with VKAs at the Washington University School of Medicine in St. Louis, as well as 86.2% of patients treated with VKAs in the historical study cohort, had mRS of 0–2 at follow-up (p = 0.60). Rates of major bleeding compared with this cohort were also similar (11.1% vs. 11.1% vs. 14.5%, p = 0.80).
Conclusions
The safety and efficacy results of NOAC use for CVT were similar to those for age-matched and sex-matched controls treated with VKAs, as well as historical published controls. Assessment of NOAC efficacy and safety in CVT in multicenter cohort studies and randomized controlled trials is warranted.
Similar content being viewed by others
References
Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352(17):1791–8.
Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162–70.
Coutinho JM, Zuurbier SM, Aramideh M, Stam J. The incidence of cerebral venous thrombosis: a cross-sectional study. Stroke. 2012;43(12):3375–7.
Devasagayam S, Wyatt B, Leyden J, Kleinig T. Cerebral venous sinus thrombosis incidence is higher than previously thought: a retrospective population-based study. Stroke. 2016;47(9):2180–2.
Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158–92.
Ferro JM, Bousser MG, Canhao P, Coutinho JM, Crassard I, Dentali F, et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis—endorsed by the European Academy of Neurology. Eur J Neurol. 2017;24(10):1203–13.
Ferro JM, Coutinho JM, Dentali F, Kobayashi A, Alasheev A, Canhao P, et al. Safety and efficacy of dabigatran etexilate versus dose-adjusted warfarin in patients with cerebral venous thrombosis: a randomized clinical trial. JAMA Neurol. 2019;76(12):1457–65.
Hon SFK, Li HLT, Cheng PW. Use of direct thrombin inhibitor for treatment of cerebral venous thrombosis. J Stroke Cerebrovasc Dis. 2012;21(8):915.e11-15.
Mendonca MD, Barbosa R, Cruz-e-Silva V, Calado S, Viana-Baptista M. Oral direct thrombin inhibitor as an alternative in the management of cerebral venous thrombosis: a series of 15 patients. Int J Stroke. 2015;10(7):1115–8.
Geisbusch C, Richter D, Herweh C, Ringleb PA, Nagel S. Novel factor Xa inhibitor for the treatment of cerebral venous and sinus thrombosis: first experience in 7 patients. Stroke. 2014;45(8):2469–71.
Anticoli S, Pezzella F, Scifoni G, Ferrari C, Pozzessere C. Treatment of cerebral venous thrombosis with rivaroxaban. J Biomedical Sci. 2016;5(3):1–2.
Covut F, Kewan T, Perez O, Flores M, Haddad A, Daw H. Apixaban and rivaroxaban in patients with cerebral venous thrombosis. Thromb Res. 2019;173:77–78.
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808.
Coutinho JM, Ferro JM, Canhao P, Barinagarrementeria F, Bousser MG, Stam J, et al. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke. 2010;41(11):2575–80.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92.
Gazioglu S, Eyuboglu I, Yildirim A, Aydin CO, Alioglu Z. Cerebral venous sinus thrombosis: clinical features, long-term outcome and recanalization. J Clin Neurosci. 2017;45:248–51.
Stolz E, Trittmacher S, Rahimi A, Gerriets T, Rottger C, Siekmann R, et al. Influence of recanalization on outcome in dural sinus thrombosis: a prospective study. Stroke. 2004;35(2):544–7.
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: chest guideline and expert panel report. Chest. 2016;149(2):315–52.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–418.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
VKSB and ARZ conceived the study idea. All authors contributed to the study protocol. JAG and VKSB undertook data collection. JAG performed the statistical analysis. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical Approval
Ethical approval for this retrospective study was granted by the Washington University School of Medicine Institutional Review Board (Ref. 201905143).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giles, J.A., Balasetti, V.K.S. & Zazulia, A.R. Non-Vitamin K Antagonist Oral Anticoagulants for the Treatment of Cerebral Venous Sinus Thrombosis: a Retrospective, Matched Cohort Analysis. Neurocrit Care 35, 783–788 (2021). https://doi.org/10.1007/s12028-021-01244-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01244-x