Abstract
Background
The objective of this study was to examine whether heart rate variability (HRV) measures can be used to detect neurocardiogenic injury (NCI).
Methods
Three hundred and twenty-six consecutive admissions with aneurysmal subarachnoid hemorrhage (SAH) met criteria for the study. Of 326 subjects, 56 (17.2%) developed NCI which we defined by wall motion abnormality with ventricular dysfunction on transthoracic echocardiogram or cardiac troponin-I > 0.3 ng/mL without electrocardiogram evidence of coronary artery insufficiency. HRV measures (in time and frequency domains, as well as nonlinear technique of detrended fluctuation analysis) were calculated over the first 48 h. We applied longitudinal multilevel linear regression to characterize the relationship of HRV measures with NCI and examine between-group differences at baseline and over time.
Results
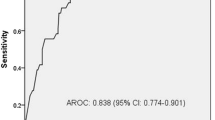
There was decreased vagal activity in NCI subjects with a between-group difference in low/high frequency ratio (β 3.42, SE 0.92, p = 0.0002), with sympathovagal balance in favor of sympathetic nervous activity. All time-domain measures were decreased in SAH subjects with NCI. An ensemble machine learning approach translated these measures into a classification tool that demonstrated good discrimination using the area under the receiver operating characteristic curve (AUROC 0.82), the area under precision recall curve (AUPRC 0.75), and a correct classification rate of 0.81.
Conclusions
HRV measures are significantly associated with our label of NCI and a machine learning approach using features derived from HRV measures can classify SAH patients that develop NCI.



Similar content being viewed by others
References
Wybraniec MT, Mizia-Stec K, Krzych L. Neurocardiogenic injury in subarachnoid hemorrhage: a wide spectrum of catecholamin-mediated brain–heart interactions. Cardiol J. 2014;21:220–8. https://doi.org/10.5603/CJ.a2014.0019.
Tung P, Kopelnik A, Banki N, et al. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke. 2004;35:548–51. https://doi.org/10.1161/01.STR.0000114874.96688.54.
Naidech AM, Kreiter KT, Janjua N, et al. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation. 2005;112:2851–6. https://doi.org/10.1161/CIRCULATIONAHA.105.533620.
Parekh N, Venkatesh B, Cross D, et al. Cardiac troponin I predicts myocardial dysfunction in aneurysmal subarachnoid hemorrhage. J Am Coll Cardiol. 2000;36:1328–35.
Banki N, Kopelnik A, Tung P, et al. Prospective analysis of prevalence, distribution, and rate of recovery of left ventricular systolic dysfunction in patients with subarachnoid hemorrhage. J Neurosurg. 2006;105:15–20. https://doi.org/10.3171/jns.2006.105.1.15.
Kothavale A, Banki NM, Kopelnik A, et al. Predictors of left ventricular regional wall motion abnormalities after subarachnoid hemorrhage. Neurocrit Care. 2006;4:199–205. https://doi.org/10.1385/NCC:4:3:199.
Malik AN, Gross BA, Rosalind Lai PM, et al. Neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2015;83:880–5. https://doi.org/10.1016/j.wneu.2015.01.013.
Lee VH, Connolly HM, Fulgham JR, et al. Tako-tsubo cardiomyopathy in aneurysmal subarachnoid hemorrhage: an underappreciated ventricular dysfunction. J Neurosurg. 2006;105:264–70. https://doi.org/10.3171/jns.2006.105.2.264.
Lee VH, Oh JK, Mulvagh SL, et al. Mechanisms in neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2006;5:243–9. https://doi.org/10.1385/NCC:5:3:243.
Kono T, Morita H, Kuroiwa T, et al. Left ventricular wall motion abnormalities in patients with subarachnoid hemorrhage: neurogenic stunned myocardium. J Am Coll Cardiol. 1994;24:636–40.
Kawai S, Suzuki H, Yamaguchi H, et al. Ampulla cardiomyopathy (‘Takotusbo’ cardiomyopathy)—reversible left ventricular dysfunction: with ST segment elevation. Jpn Circ J. 2000;64:156–9.
Zaroff JG, Rordorf GA, Ogilvy CS, et al. Regional patterns of left ventricular systolic dysfunction after subarachnoid hemorrhage: evidence for neurally mediated cardiac injury. J Am Soc Echocardiogr. 2000;13:774–9.
Murthy SB, Shah S, Rao CP, et al. Neurogenic stunned myocardium following acute subarachnoid hemorrhage: pathophysiology and practical considerations. J Intensive Care Med. 2015;30:318–25. https://doi.org/10.1177/0885066613511054.
Kilbourn KJ, Levy S, Staff I, et al. Clinical characteristics and outcomes of neurogenic stress cadiomyopathy in aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg. 2013;115:909–14. https://doi.org/10.1016/j.clineuro.2012.09.006.
Temes RE, Tessitore E, Schmidt JM, et al. Left ventricular dysfunction and cerebral infarction from vasospasm after subarachnoid hemorrhage. Neurocrit Care. 2010;13:359–65. https://doi.org/10.1007/s12028-010-9447-x.
Kilbourn KJ, Ching G, Silverman DI, et al. Clinical outcomes after neurogenic stress induced cardiomyopathy in aneurysmal sub-arachnoid hemorrhage: a prospective cohort study. Clin Neurol Neurosurg. 2015;128:4–9. https://doi.org/10.1016/j.clineuro.2014.10.017.
Bulsara KR, McGirt MJ, Liao L, et al. Use of the peak troponin value to differentiate myocardial infarction from reversible neurogenic left ventricular dysfunction associated with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;98:524–8. https://doi.org/10.3171/jns.2003.98.3.0524.
Spann JF Jr, Moellering RC Jr, Haber E, et al. Arrhythmias in acute myocardial infarction; a study utilizing an electrocardiographic monitor for automatic detection and recording of arrhythmias. N Engl J Med. 1964;271:427–31. https://doi.org/10.1056/NEJM196408272710901.
Julian DG, Valentine PA, Miller GG. Disturbances of rate, rhythm and conduction in acute myocardial infarction: a prospective study of 100 consecutive unselected patients with the aid of electrocardiographic monitoring. Am J Med. 1964;37:915–27.
Stock E, Goble A, Sloman G. Assessment of arrhythmias in myocardial infarction. Br Med J. 1967;2:719–23.
Moorman JR, Delos JB, Flower AA, et al. Cardiovascular oscillations at the bedside: early diagnosis of neonatal sepsis using heart rate characteristics monitoring. Physiol Meas. 2011;32:1821–32. https://doi.org/10.1088/0967-3334/32/11/S08.
Binici Z, Mouridsen MR, Kober L, et al. Decreased nighttime heart rate variability is associated with increased stroke risk. Stroke. 2011;42:3196–201. https://doi.org/10.1161/STROKEAHA.110.607697.
Ryan ML, Ogilvie MP, Pereira BM, et al. Heart rate variability is an independent predictor of morbidity and mortality in hemodynamically stable trauma patients. J Trauma. 2011;70:1371–80. https://doi.org/10.1097/TA.0b013e31821858e6.
Mazzeo AT, La Monaca E, Di Leo R, et al. Heart rate variability: a diagnostic and prognostic tool in anesthesia and intensive care. Acta Anaesthesiol Scand. 2011;55:797–811. https://doi.org/10.1111/j.1399-6576.2011.02466.x.
DeGiorgio CM, Miller P, Meymandi S, et al. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: the SUDEP-7 Inventory. Epilepsy Behav. 2010;19:78–81. https://doi.org/10.1016/j.yebeh.2010.06.011.
Park S, Kaffashi F, Loparo KA, et al. The use of heart rate variability for the early detection of treatable complications after aneurysmal subarachnoid hemorrhage. J Clin Monit Comput. 2013;27:385–93. https://doi.org/10.1007/s10877-013-9467-0.
Waldenborg M, Soholat M, Kahari A, et al. Multidisciplinary assessment of tako tsubo cardiomyopathy: a prospective case study. BMC Cardiovasc Disord. 2011;11:14. https://doi.org/10.1186/1471-2261-11-14.
Deibert E, Barzilai B, Braverman AC, et al. Clinical significance of elevated troponin I levels in patients with nontraumatic subarachnoid hemorrhage. J Neurosurg. 2003;98:741–6. https://doi.org/10.3171/jns.2003.98.4.0741.
Mayer SA, LiMandri G, Sherman D, et al. Electrocardiographic markers of abnormal left ventricular wall motion in acute subarachnoid hemorrhage. J Neurosurg. 1995;83:889–96. https://doi.org/10.3171/jns.1995.83.5.0889.
Syed TU, Kaffashi F, Loparo KA, et al. System, apparatus and method for diagnosing seizures. 2014.
Camm AJ, et al. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–81.
Peng CK, Buldyrev SV, Havlin S, et al. Mosaic organization of DNA nucleotides. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1994;49:1685–9.
Johnson AE, Ghassemi MM, Nemati S, et al. Machine learning and decision support in critical care. Proc IEEE Inst Electr Electron Eng. 2016;104:444–66. https://doi.org/10.1109/JPROC.2015.2501978.
Bzdok D, Krzywinski M, Altman N. Points of Significance: Machine learning: a primer. Nat Methods. 2017;14:1119–1120
Rickards CA, Ryan KL, Ludwig DA, et al. Is heart period variability associated with the administration of lifesaving interventions in individual prehospital trauma patients with normal standard vital signs? Crit Care Med. 2010;38:1666–73. https://doi.org/10.1097/CCM.0b013e3181e74cab.
Peng H, Long F, Ding C. Feature selection based on mutual information: criteria of max-dependency, max-relevance, and min-redundancy. IEEE Trans Pattern Anal Mach Intell. 2005;27:1226–38. https://doi.org/10.1109/TPAMI.2005.159.
Davis J, Goadrich M. The relationship between precision–recall and ROC curves. In: Proceedings of the 23rd international conference on machine learning, Pittsburgh, Pennsylvania, USA: ACM; 2006. p. 233–40.
Claesen M, Smet FD, Suykens JAK, et al. A robust ensemble approach to learn from positive and unlabeled data using SVM base models. Neurocomputing. 2015;160:73–84. https://doi.org/10.1016/j.neucom.2014.10.081.
Goadrich M, Oliphant L, Shavlik J. Gleaner: creating ensembles of first-order clauses to improve recall-precision curves. Mach Learn. 2006;64:231–61. https://doi.org/10.1007/s10994-006-8958-3.
Leisman DE. Rare events in the ICU: an emerging challenge in classification and prediction. Crit Care Med. 2018;46:418–24. https://doi.org/10.1097/CCM.0000000000002943.
Mosley WJ 2nd, Manuchehry A, McEvoy C, et al. Takotsubo cardiomyopathy induced by dobutamine infusion: a new phenomenon or an old disease with a new name. Echocardiography. 2010;27:E30–3. https://doi.org/10.1111/j.1540-8175.2009.01089.x.
Saito R, Takahashi T, Noshita N, et al. Takotsubo cardiomyopathy induced by dobutamine infusion during hypertensive therapy for symptomatic vasospasm after subarachnoid hemorrhage—case report. Neurol Med Chir (Tokyo). 2010;50:393–5.
Taccone FS, Brasseur A, Vincent JL, et al. Levosimendan for the treatment of subarachnoid hemorrhage-related cardiogenic shock. Intensive Care Med. 2013;39:1497–8. https://doi.org/10.1007/s00134-013-2945-5.
Santoro F, Ieva R, Ferraretti A, et al. Safety and feasibility of levosimendan administration in takotsubo cardiomyopathy: a case series. Cardiovasc Ther. 2013;31:e133–7. https://doi.org/10.1111/1755-5922.12047.
Yaman M, Arslan U, Kaya A, et al. Levosimendan accelerates recovery in patients with takotsubo cardiomyopathy. Cardiol J. 2016;23:610–5. https://doi.org/10.5603/CJ.a2016.0100.
Mayer SA, Lin J, Homma S, et al. Myocardial injury and left ventricular performance after subarachnoid hemorrhage. Stroke. 1999;30:780–6.
Kawahara E, Ikeda S, Miyahara Y, et al. Role of autonomic nervous dysfunction in electrocardio-graphic abnormalities and cardiac injury in patients with acute subarachnoid hemorrhage. Circ J. 2003;67:753–6.
Gail MH, Pfeiffer RM. Breast cancer risk model requirements for counseling, prevention, and screening. J Natl Cancer Inst. 2018;110:994–1002. https://doi.org/10.1093/jnci/djy013.
Saria S, Rajani AK, Gould J, et al. Integration of early physiological responses predicts later illness severity in preterm infants. Sci Transl Med. 2010;2:48ra65. https://doi.org/10.1126/scitranslmed.3001304.
Mani S, Ozdas A, Aliferis C, et al. Medical decision support using machine learning for early detection of late-onset neonatal sepsis. J Am Med Inform Assoc JAMIA. 2014;21:326–36. https://doi.org/10.1136/amiajnl-2013-001854.
Gultepe E, Green JP, Nguyen H, et al. From vital signs to clinical outcomes for patients with sepsis: a machine learning basis for a clinical decision support system. J Am Med Inform Assoc JAMIA. 2014;21:315–25. https://doi.org/10.1136/amiajnl-2013-001815.
Nachimuthu SK, Haug PJ. Early detection of sepsis in the emergency department using dynamic bayesian networks. AMIA Annu Symp Proc. 2012;2012:653–62.
Henry KE, Hager DN, Pronovost PJ, et al. A targeted real-time early warning score (TREWScore) for septic shock. Sci Transl Med. 2015;7:299ra122. https://doi.org/10.1126/scitranslmed.aab3719.
Calvert JS, Price DA, Chettipally UK, et al. A computational approach to early sepsis detection. Comput Biol Med. 2016;74:69–73. https://doi.org/10.1016/j.compbiomed.2016.05.003.
Stanculescu I, Williams CK, Freer Y. Autoregressive hidden Markov models for the early detection of neonatal sepsis. IEEE J Biomed Health Inform. 2014;18:1560–70. https://doi.org/10.1109/JBHI.2013.2294692.
Tang CH, Middleton PM, Savkin AV, et al. Non-invasive classification of severe sepsis and systemic inflammatory response syndrome using a nonlinear support vector machine: a preliminary study. Physiol Meas. 2010;31:775–93. https://doi.org/10.1088/0967-3334/31/6/004.
Taylor RA, Pare JR, Venkatesh AK, et al. Prediction of in-hospital mortality in emergency department patients with sepsis: a local big data-driven, machine learning approach. Acad Emerg Med. 2016;23:269–78. https://doi.org/10.1111/acem.12876.
Ong ME, Lee Ng CH, Goh K, et al. Prediction of cardiac arrest in critically ill patients presenting to the emergency department using a machine learning score incorporating heart rate variability compared with the modified early warning score. Crit Care. 2012;16:R108. https://doi.org/10.1186/cc11396.
Roederer A, Holmes JH, Smith MJ, et al. Prediction of significant vasospasm in aneurysmal subarachnoid hemorrhage using automated data. Neurocrit Care. 2014;21:444–50. https://doi.org/10.1007/s12028-014-9976-9.
Mayer SA, Fink ME, Homma S, et al. Cardiac injury associated with neurogenic pulmonary edema following subarachnoid hemorrhage. Neurology. 1994;44:815–20.
Pollick C, Cujec B, Parker S, et al. Left ventricular wall motion abnormalities in subarachnoid hemorrhage: an echocardiographic study. J Am Coll Cardiol. 1988;12:600–5.
Sato K, Masuda T, Kikuno T, et al. Left ventricular asynergy and myocardial necrosis accompanied by subarachnoid hemorrhage: contribution of neurogenic pulmonary edema. J Cardiol. 1990;20:359–67.
Yamaguchi T, Shimizu Y, Ono N, et al. A case of subarachnoid hemorrhage with electrocardiographic and echocardiographic changes simulating transmural myocardial infarction. Jpn J Med. 1991;30:142–5.
Hillebrand S, Gast KB, de Mutsert R, et al. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace. 2013;15:742–9. https://doi.org/10.1093/europace/eus341.
Rickards CA, Ryan KL, Convertino VA. Characterization of common measures of heart period variability in healthy human subjects: implications for patient monitoring. J Clin Monit Comput. 2010;24:61–70. https://doi.org/10.1007/s10877-009-9210-z.
Ryan KL, Rickards CA, Ludwig DA, et al. Tracking central hypovolemia with ecg in humans: cautions for the use of heart period variability in patient monitoring. Shock. 2010;33:583–9. https://doi.org/10.1097/SHK.0b013e3181cd8cbe.
Sethuraman G, Ryan KL, Rickards CA, et al. Ectopy in trauma patients: cautions for use of heart period variability in medical monitoring. Aviat Space Environ Med. 2010;81:125–9.
Hinojosa-Laborde C, Rickards CA, Ryan KL, et al. Heart rate variability during simulated hemorrhage with lower body negative pressure in high and low tolerant subjects. Front Physiol. 2011;2:85. https://doi.org/10.3389/fphys.2011.00085.
Salomao E Jr, Otsuki DA, Correa AL, et al. Heart rate variability analysis in an experimental model of hemorrhagic shock and resuscitation in pigs. PLoS ONE. 2015;10:e0134387. https://doi.org/10.1371/journal.pone.0134387.
Ryan ML, Thorson CM, Otero CA, et al. Clinical applications of heart rate variability in the triage and assessment of traumatically injured patients. Anesthesiol Res Pract. 2011;2011:416590. https://doi.org/10.1155/2011/416590.
Sacha J, Pluta W. Alterations of an average heart rate change heart rate variability due to mathematical reasons. Int J Cardiol. 2008;128:444–7. https://doi.org/10.1016/j.ijcard.2007.06.047.
Author Contributions
KT, MM, SP, SM, AV, ES, DR, SA, and JC contributed to data collection; MM, SP, AB, and FK performed the analysis; KT, MM, and SP contributed to writing; all authors contributed equally to editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Source of support
This study was funded by National Institute of Health (NIH), Grant Number: NIEHS K01-ES026833-02 (SP).
Conflict of interest
None.
Ethical approval/informed consent
The study was approved by the Columbia University Medical Center Institutional Review Board. In all cases, written informed consent was obtained from the patient or a surrogate.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Megjhani, M., Kaffashi, F., Terilli, K. et al. Heart Rate Variability as a Biomarker of Neurocardiogenic Injury After Subarachnoid Hemorrhage. Neurocrit Care 32, 162–171 (2020). https://doi.org/10.1007/s12028-019-00734-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00734-3