Abstract
Background
In many neurological diseases, intracranial pressure (ICP) is elevated and needs to be actively managed. ICP is typically measured with an invasive transducer, which carries risks. Non-invasive techniques for monitoring ICP (nICP) have been developed. The aim of this study was to compare three different methods of transcranial Doppler (TCD) assessment of nICP in an animal model of acute intracranial hypertension.
Methods
In 28 rabbits, ICP was increased to 70–80 mmHg by infusion of Hartmann’s solution into the lumbar subarachnoid space. Doppler flow velocity in the basilar artery was recorded. nICP was assessed through three different methods: Gosling’s pulsatility index PI (gPI), Aaslid’s method (AaICP), and a method based on diastolic blood flow velocity (FVdICP).
Results
We found a significant correlation between nICP and ICP when all infusion experiments were combined (FVdICP: r = 0.77, AaICP: r = 0.53, gPI: r = 0.54). The ability to distinguish between raised and ‘normal’ values of ICP was greatest for FVdICP (AUC 0.90 at ICP >40 mmHg). When infusion experiments were considered independently, FVdICP demonstrated again the strongest correlation between changes in ICP and changes in nICP (mean r = 0.85).
Conclusions
TCD-based methods of nICP monitoring are better at detecting changes of ICP occurring in time, rather than absolute prediction of ICP as a number. Of the studied methods of nICP, the method based on FVd is best to discriminate between raised and ‘normal’ ICP and to monitor relative changes of ICP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elevated intracranial pressure (ICP) is an important secondary brain injury because it can cause both brain ischaemia and mechanical compression of vital brain structures. Unsurprisingly, intracranial hypertension is associated with a poor outcome [1]. Its evaluation is crucial in many neurological diseases [2, 3] as clinical signs of elevated ICP (such as altered consciousness, headache, vomiting) are not always reliable predictors of this condition [4].
Invasive measurement of ICP through an intraventricular or intraparechymal catheter is considered the gold standard [5].
However, these methods can cause complications such as infection, hemorrhage, or soft tissue scarring [6–9]. Non-invasive monitoring of ICP could be valuable in the management of many neurological patients. Several techniques have been proposed for the non-invasive measurement of ICP [10–12], but none of these seem to be accurate enough to be used as a replacement for invasive ICP measurement.
Transcranial Doppler ultrasonography (TCD) is one possible tool to assess non-invasive ICP (nICP) and cerebral perfusion pressure (CPP). Increased ICP produces specific changes in cerebral blood flow velocity (FV) measured by TCD, with diastolic FV being particularly sensitive [13]. Gosling pulsatility index (gPI) was one of the first measures derived from the TCD waveform that has been studied in relation to ICP [14–16]. However, its clinical utility is questionable due to its poor precision [17, 18].
Other formulae and mathematical approaches that incorporate the arterial blood pressure as well as the cerebral blood flow velocity waveform have been proposed. Aaslid et al. [19, 20] presented a formula for the estimation of CPP, based on spectral pulsatility index (SPI) and the first harmonic component of the arterial blood pressure (ABP) which demonstrated to be quite sensitive to the variation of CPP, but it has a limited accuracy [21]. Czosnyka et al. proposed a similar but modified formula for non-invasive estimation of CPP (nCPP), and therefore of ICP, which showed a low estimation error compared to real CPP [22].The authors reinforced these preliminary results in a prospective study demonstrating that the absolute difference between real CPP and nCPP was <10 mmHg in 89 % of measurements and <13 mmHg in 92 % of measurements with a confidence range for predictors that was no wider than ±12 mmHg [23]. Whether these formulae for estimating CPP can be used to assess ICP non-invasively by simple mathematical rearrangement (i.e., nICP = ABP − nCPP) is unclear.
The aim of this study was to compare the ability of these three non-invasive methods [20, 22, 28] to monitor experimental raise in ICP.
Methods
All the animal experiments performed in this study were carried out in 1995 under UK Home Office license, with permission from the Institutional Animal Care and Use Committee at Cambridge University and in accordance with the standards set by the UK Animals Scientific Procedures Act of 1986. Recordings from 28 experiments of male New Zealand White rabbits (weighing from 2.7 to 3.7 kg) were analyzed retrospectively, including high-resolution sampling of ABP, ICP, and basilar artery FV. The experimental protocols have been previously presented [24–26] and briefly described here.
A tracheostomy was performed, and a jugular vein was cannulated. A femoral artery was cannulated and the catheter advanced to lie high in the dorsal aorta to monitor arterial blood pressure (GaelTec, Dunvegan, UK) and for blood gas analysis. The animals were placed on a padded warming blanket, and the rectal temperature was monitored. A general anesthesia was induced using intravenous alphaxalone/alphadalone (Saffan, Pitman-Moore, Uxbridge, UK, 0.2 mL/kg). Halothane at 1.5 % in 3:1 nitrous oxide/oxygen was maintained. FV was monitored with an 8 MHz pulsed Doppler ultrasound probe (PcDop 842, SciMed, Bristol, UK) positioned over the basilar artery. To access the artery, a posterior frontal burr hole (7-mm diameter) at the bregma to the left of the midline was made and the Doppler ultrasound probe was positioned over the exposed dura and adjusted to obtain the best Doppler spectra from the basilar artery. Two weeks before experiment both carotid arteries of the rabbits were ligated (on different days), to make cerebral blood flow dependent on basilar artery flow. A second burr hole of diameter 1.5 mm was made over the contralateral cerebral hemisphere close to the bregma, and a Laser Doppler probe of 1 mm in diameter (Moor Instruments, Axbridge, Devon, UK) was placed epidurally. ICP was monitored through a third burr hole 1.5 mm in diameter, after insertion of an intraparenchymal microsensor (Codman and Shurtleff, Raynham, MA, USA).
Ventilation was controlled and PaCO2 monitored and stabilized adjusting the ventilator tidal volume (confirmed by blood gas analysis) to normocapnia. Parameters were compared at baseline ICP and ABP. A laminectomy was performed in all the rabbits, and a catheter was inserted into the lumbar subarachnoid space. After recovery from surgery and stabilization of PaCO2, ABP, ICP, basilar artery FV and Laser Doppler Flowmetry LDF values were recorded to establish a baseline before experimental maneuvers.
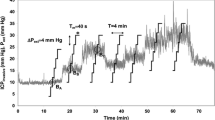
ICP was increased by infusion of Hartmann’s solution into the lumbar catheter in the subarachnoid space at an increasing rate from 0.1 to 2 mL/min to produce a controlled and marked rise in ICP. ABP, CPP, and FV were continuously recorded, converted into digital samples using an analog-to-digital converter fitted into an IBM compatible personal computer [27] and saved on hard disk in digital form, with sampling frequency 50 Hz.
Data Acquisition and Analysis
The recorded signals were analyzed using our own software for clinical data processing (ICM+; http://www.neurosurg.cam.ac.uk/icmplus). The maximal and minimal values of ABP and FV from consecutive 2 s epochs were derived and then averaged over 10 s to give ABPs, ABPd, FVs, and FVd, respectively. Fundamental amplitudes of FV and ABP pulse waveforms were calculated using spectral analysis. Heart rate was calculated using position of the spectral peak associated with the first harmonic of ABP. Mean values of ABP, ICP, FV, and CPP (and non-invasive indicators of ICP described below) were calculated in 30 s epochs by ICM plus.
Calculation of CPP and ICP
For each rabbit, we calculated non-invasive CPP and consequently non-invasive ICP according to the following three formulas.
PI was calculated according to the method of Gosling [28]:
where FVs is the systolic flow velocity, FVd the diastolic flow velocity, and FVm is the mean flow velocity.
nICP derived by gPI (gPIICP) was calculated according to a formula based on data analyzed by Budohoski et al. [29].
According to Aaslid formula [20], nCPP is derived from spectral pulsatility index (SPI). SPI is obtained by the first harmonic component of flow velocity pulsation (FV1), divided by the FVm (SPI = FV1/FVm). nCPP according to Aaslid Formula (aCPP) is derived from the relationship between the first harmonic component of the pressorial pulse (A1) and SPI.
According to Czosnyka et al. [22] (dCPP), nCPP has been calculated as
\( {\text{dCPP}} = {\text{ABPm}}*{\text{FVd}}/{\text{FVm}} + 1 4 \), where ABPm is defined as the mean arterial blood pressure.
Finally, ICP is then estimated as the difference between inflow (MAP) and CPP:
Cushing Response Assessment
Cushing response (CR) was defined according to previous description [30] as a compensatory baroreceptor response to critical ICP increases, aiming to keep adequate values of CPP consisting of a rise in systolic blood pressure, a reduction in flow velocity, and increase of heart rate variance after critical increase of ICP [31]. According to this definition, we identified in each rabbits two periods (before CR, pre CR, and during CR) and three different periods of infusion test (T0 = baseline, before the beginning of infusion test; T1 = when CR begins; T2 = maximum peak of ICP), and we assessed the influence of CR on accuracy of nICP monitoring calculating correlation with measured ICP, BIAS, and 95 % CI (mmHg) within every rabbit for each formula.
Statistics
The relationship between measured ICP and each non-invasive estimate was compared within each rabbit using the Pearson correlation coefficient using a combination of Microsoft excel (Microsoft Corp., Redmond, USA) and ICM+ tools (Monash University, Melbourne, Australia). The Bland–Altman method was used for calculation of bias and 95 % CI for prediction of ICP. Receiver operating characteristic (ROC) curve analyses were performed to determine performance of each non-invasive ICP method in distinguishing between elevated and ‘normal’ values of ICP set arbitrarily as cut points of 20 and 40 mmHg. The ROC curve was created by plotting the true-positive rate (sensitivity) against the corresponding false-positive rate (1-specificity) for different cut-off points of a parameter [32] (in this case ICP). The area under the curve (AUC) is a measure of how well the three methods can detect elevation of ICP above (or decrease of CPP below) given threshold for which the curve was constructed. In this ROC analysis, each 30 s data point was considered as an independent. ROC analysis was performed using IBM SPSS version 21.0 software (IBM Corp., Armonk, USA).
Results
Correlation Analyses
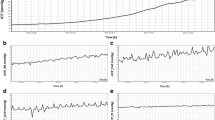
Examples of the recordings and nICP monitoring in single rabbit are shown in Fig. 1.
Traces displaying examples of experiments for each formula. In all the examples, it is shown progressive intracranial hypertension owing to infusion of Hartmann’s solution into the lumbar subarachnoid space and corresponding non-invasive intracranial pressure (nICP) calculated with FVdICP and Aaslid formula. a An example of good correlation between ICP and nICP in a single rabbit. From top to the bottom, invasive ICP and then nICP measured with FVdICP, and Aaslid formula. b An example of bad correlations of ICP in a single rabbit with the two methods
Scatter plots of 30 s averaged measured ICP versus nICP from all 10-s samples pooled from all rabbits (n = 661) are shown in Fig. 2. The three formulae showed a statistically significant positive relationship with measured ICP. FVdICP showed the best correlation (r = 0.77). Aaslid formula showed a correlation with ICP of r = 0.53. Gosling PI was also correlated with ICP (r = 0.54).
Scatter plots of 30 s averaged measured ICP versus AaICP (left panel), gPI (middle panel), and FVdICP (right panel). Linear regression and relationship of multiple points from all rabbits combined (n = 1344). Each of the three nICP estimators showed a statistically significant positive relationship with measured ICP
Relative Assessment of nICP
Among the individual recordings, there were cases in which nICP and ICP showed very high correlation and those in which a nICP and ICP correlation was rather poor. In two cases, the correlation coefficient was worse than 0.6 for all the three methods. Some examples are provided in Fig. 1. To assess the ability to non-invasively estimate relative changes in ICP, correlation coefficients between measured ICP and each non-invasive estimate of ICP were performed in each experiment. The best method to detect changes nICP seems to be FVdICP (mean r = 0.85), followed by AaICP (mean r = 0.61) and gPI (mean r = 0.32) as shown in Table 1.
Predictive Ability of nICP Methods
We assessed the 95 % CI and the bias (nICP-ICP) during each infusion experiment using Bland–Altman methodology. Results are described in Table 1. The 95 % CI for prediction was on average 26.26 mmHg for FVdICP, 59.6 mmHg for Aa formula, and 38.56 mmHg for gPI with the smallest bias resulted from FVdICP method (−1.39 ± 11.86 mmHg SD).
Results of receiver operating characteristic (ROC) analysis with outcomes dichotomised as ICP above or below certain thresholds are presented in Fig. 3. We assessed the predictive ability of each non-invasive ICP method using a cut-off at 20 and 40 mmHg. At cut-off at 20 mmHg, FVdICP showed the highest area under the curve (AUC) = 0.86 compared to the other methods (AaICP AUC = 0.66, gPI AUC = 0.62). Using a cut-off at 40 mmHg, FVdICP showed again the highest AUC compared to the other formula (FVdICP AUC = 0.94, AaICP AUC = 0.77, gPI = 0.66 (Fig. 3).
nICP methods receiver operating characteristic (ROC) analysis plot representing specificity and sensitivity of FVdICP, AaICP, gPI, dichotomized as ICP above or below two thresholds, 20 and 40 mmHg. These results suggest that FVdICP is the best method to distinguish between elevated and non-elevated ICP
Effects of Cushing Response in the Accuracy of ICP Measurement
Before the beginning of CR, the three methods show lower correlation coefficients with invasive ICP compared to the whole monitoring results: 0.32 for FVdICP, 0.07 for AaICP, and 0.12 for gPIICP. In the same period, the results of Bland and Altman analysis between nICP methods and ICP reported a −8.3 mmHg bias with a 95 % CI of ±18.1 for FVdICP; −31.06 mmHg with a 95 % CI of ±42.2 for AaICP and −1.88 mmHg with a 95 % CI of ±20.36 for gPIICP.
During CR, all the three methods show a better correlation with invasive ICP: r = 0.6 for FVdICP, 0.34 for Aaslid formula, and 0.22 for gPIICP. On this dataset, we calculated a bias of −10.94 mmHg with ±12.88 95 % CI values for FVdICP; a bias of −11.04 mmHg with a ±18.84 95 % CI for Aa formula, and a bias of −57.41 with ±12 95 % CI for gPIICP.
FVdICP method showed a statistically significant correlation in all the considered points of measurement, with highest coefficient correlation for ΔICP between T2 and T1 (ΔT2 − T1: r = 0.73, p = 0.00001; ΔT1 − T0: r = 0.44, p = 0.02; ΔT2 − T0: r = 0.68, p = 0.00007). Aaslid method demonstrated a significant correlation with ICP in the difference between T2 and T1 and T2 − T0 (ΔT2 − T1: r = 0.57, p = 0.001; ΔT2 − T0: r = 0.66, p = 0.0001) and a non-significative correlation between T0 and T1 (ΔT1 − T0: r = 0.21, p = 0.26). ΔgPIICP was not significantly correlated to ΔICP in any of these points (ΔT2 − T0, r = 0.28, p = 0.14; ΔT1 − T0, r = 0.02, p = 0.90; ΔT1 − T2, r = 0.41 p = 0.03).
Discussion
An active management of ICP is necessary in many neurological and neurosurgical diseases [3, 33]. Moreover, non-invasive estimation of ICP can be useful in patients at risk from intracranial hypertension when coagulation abnormalities prevent insertion of invasive ICP devices. Many attempts have been made to find an “estimator” for ICP [34–38].
TCD is an attractive option as it is safe with no reports of technique-related complications and it is suitable for continuous monitoring of relative changes of ICP happening in time. In this study, we compared the reliability of three different formulas TCD based for the estimation of nICP (gPI [28], Aaslid formula [19] and FVd formula [22]) in an animal model where an acute intracranial hypertension was induced.
We found a statistically significant positive relationship between all nICP and ICP when each time point from each infusion is considered as an independent sample. However, the scatter seems unacceptably high, showing wide 95 % confidence intervals for prediction for the three methods. The most promising method to assess absolute values of ICP seems to be the method based on diastolic blood flow velocity (r = 0.77) (Fig. 2).
Better results are obtained if we consider relative changes in ICP within each infusion experiment. Also in this case, FVdICP formula shows the best correlation with ICP with a r = 0.85 (Table 1). The variability of the correlations between ICP and nICP within each infusion experiment could be due to occasional suboptimal quality of the Doppler signals in some experiments. Thus, in some infusion tests we observed a plot with good correlation, while in others we found poor correlations using the three formulas (Fig. 1).
The results obtained suggest that the three methods are not accurate in the absolute measurement of ICP. Indeed, biases calculation showed tendency to underestimate real ICP (Table 1) and 95 % CI values were large in all the cases. However, these methods could be very helpful to detect relative changes of ICP within individual cases. In particular, FVdICP formula showed the best correlation with invasive ICP for relative values of ICP (Table 1), and the best performance at distinguishing between elevated and non-elevated ICP at a cut-off at 20 and 40 mmHg as for ROC analysis results (Fig. 3). This indicates that inclusion of ABP in addition to FV into the model of non-invasive ICP improves performance.
Generally, the accuracy of detection of changes in ICP seems to improve at very high levels of ICP (during CR) with better correlation coefficients and smaller 95 % CI. The better results that we obtained during CR are probably due to the fact that CR involves many haemodynamic modifications (in particular ABP) which could influence the methods, in particular FVdICP formula. However, it is difficult to state for sure that CR itself is a real factor, or rather mean level of ICP. The same analysis may be interpreted that nICP methodology performs better when ICP is truly elevated.
In addition, FVdICP formula is the best method to detect the magnitude of changes of ICP, at different levels of ICP, from physiological values (T0, baseline) to pathophysiological values of ICP to very critical values of ICP (T1 and T2), as shown in Table 2.
The results obtained suggest that non-invasive ICP TCD methods are not yet accurate enough for absolute assessment of ICP and cannot replace invasive ICP altogether. However, they may still be useful in specific clinical scenarios. FVdICP, for example, could have a role to help for the decision to insert a wire on admission to the Emergency Department or in Intensive Care Unit, or for monitoring changes of ICP in an individual patient even after a wire has been taking out. Also, it could be a good option in conditions in which an invasive monitoring is not indicated but a monitoring of ICP is desirable, such as liver failure, cardiac arrest, or organ transplants. The mobility of the equipment, the possibility of repeated bedside investigations and the non-invasive nature of the technique, makes TCD a device of potential benefit for monitoring brain perfusion and ICP when the direct measurement is not available or not indicated [39].
Limitations
There are some considerations that deserve to be mentioned as it is difficult to judge which method is better at detecting ICP in an animal model in which intracranial hypertension is acutely induced by infusion of liquid into CSF space. Recordings of acute intracranial hypertension due to increased CSF circulation [40] are not common scenarios in humans and in neurocritical care patients (except particular cases such as acute hydrocephalus for ventricular shunt or drain obstruction) and literature is lacking in terms of nICP estimation during CR [41, 42]. Therefore, the pathophysiological mechanisms underlying raise in ICP are quite different in “in vivo” brain injured patients.
Subsequently, the mechanism of ICP elevation in our study (continuous CSF infusion) and the hemodynamic variations associated with CR [40, 41] could contribute to the variance observed from human studies in which intracranial hypertension is associated more commonly with cerebral oedema or hemorrhage. In particular, gPI has been proven [17] to be a complex function of many mutually interdependent hemodynamic parameters and this could explain the poor correlation that we found in our model. Moreover, the clinical applications of this model can partially be disturbed by clamping carotid arteries, a procedure used to make CBF completely dependent on basilar artery flow; this may affect the correlation between nICP and ICP in real clinical situations.
The present study was performed using digitally recorded previous experiments [24–26]. However, we believe that such a ‘recycling’ of existing data using new calculation methods is not only scientifically valid but also ethically welcome as it reduces the need for new animal studies.
Conclusions
In our animal model of acute intracranial hypertension, the three TCD-based nICP methods showed better performance at detecting changes of ICP within an animal, rather than absolute prediction of ICP. According to our results and in agreement with previous studies, we think that invasive ICP monitoring may not yet be replaced with non-invasive methods. However, a real-time assessment of ICP with a non-invasive method may indicate gross intracranial hypertension and help in detection of gradual intracranial hypertension over time. Further clinical studies and critical appraisal of different non-invasive methods to estimate ICP on humans are needed to clarify the clinical reliability of these techniques.
References
Marmarou A, Anderson RL, Ward JD, Young HF, Marmarou A. Impact of ICP instability and hypotension on outcome in patients with severe head trauma. J Neurosurg. 1991;75:S59–66.
Miller JD, Becker DP, Ward JD, Sullivan HG, Adams WE, Rosner MJ. Significance of intracranial hypertension in severe head injury. J Neurosurg. 1977;47:503–616.
Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry. 2004;75:813–21.
Tayal VS, Neulander M, Norton HJ, Foster T, Saunders T, Blaivas M. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. 2007;49:508–14.
Brain Trauma Foundation. American Association of Neurological Surgeons (AANS), Congress of Neurological Surgeons (CNS), AANS/CNS Joint Section on Neuro- trauma and Critical Care. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007;24(Suppl 1):S37–44.
Holloway KL, Barnes T, Choi S, et al. Ventriculostomy infections: the effect of monitoring duration and catheter exchange in 584 patients. J Neurosurg. 1996;85:419–24.
Hoefnagel D, Dammers R, Ter Laak-Poort MP, Avezaat CJ. Risk factors for infections related to external ventricular drainage. Acta Neurochir (Wien). 2008;150:209–14.
Binz DD, Toussaint LG III, Friedman JA. Hemorrhagic complications of ventriculostomy placement: a meta-analysis. Neurocrit Care. 2009;10:253–6.
Bauer DF, Razdan SN, Bartolucci AA, Markert JM. Meta-analysis of hemorrhagic complications from ventriculostomy placement by neurosurgeons. Neurosurgery. 2011;69:255–60.
Kirk T, Jones K, Miller S, Corbett J. Measurement of intraocular and intracranial pressure:is there a relationship? Ann Neurol. 2011;70(2):323–6.
Shimbles S, Dodd C, Banister K, Mendelow AD, Chambers IR. Clinical comparison of tympanic membrane displacement with invasive ICP measurements. Acta Neurochir Suppl. 2005;95:197–9.
Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Utrasonography of optic nerve sheet diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37(7):1059–68.
Czosnyka M, Richards HK, Whitehouse HE, Pickard JD. Relationship between transcranial Doppler-determined pulsatility index and cerebrovascular resistance: an experimental study. J Neurosurg. 1996;84(1):79–84.
Brandi G, Brandi M, Sailer S, Haberther C, Stocker R, Stover JF. Transcranial color-coded duplex sonography allows to assess cerebral perfusion pressure noninvasively following severe traumatic brain injury. Acta Neurochir (Wien). 2010;152(6):965–72.
Bellner J, Romner B, Reinstrup P, Kristiansson KA, Ryding E, Brandt L. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP). Surg Neurol. 2004;62(1):45–51.
Melo JR, DiRocco F, Blanot S, et al. Transcranial Doppler can predict intracranial hypertension in children with severe traumatic brain injuries. Childs Nerv Syst. 2011;27(6):979–84.
De Riva N, Budohoski KP, Smielewski P, et al. Transcranial Doppler pulsatility index: what it is and what it isn’t. Neurocrit Care. 2012;17(1):58–66.
Zweifel C, Czosnyka M, Carrera E, De Riva N, Pickard JD, Smielewski P. Reliability of the blood flow velocity pulsatility index for assessment of intracranial and cerebral perfusion pressures in head-injured patients. Neurosurgery. 2012;71:853–61.
Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–857.
Aaslid R, Lundar T, Lindegaard KF, et al. Estimation of cerebral perfusion pressure from arterial blood pressure and transcranial Doppler recordings. In: Miller JD, Teasdale GM, Rowan JO, editors. Intracranial Pressure VI. Berlin: Springer; 1986. p. 226–32.
Czosnyka M, Kirkpatrick P, Guazzo E, et al. Can tcd pulsatility indices be used for a non invasive assessment of cerebral perfusion pressure in head injured patients? In: Naagai H, Kamiya K, Ishii S, editors. Intracranial Pressure IX. Tokyo: Springer; 1994. p. 149.
Czosnyka M, Matta B, Smielewski P, Kirkpatrick PJ, Pickard JD. Cerebral perfusion pressure in head-injured patients: a noninvasive assessment using transcranial Doppler ultrasonography. J Neurosurg. 1998;88:802–8.
Schmidt EA, Czosnyka M, Gooskens I, et al. Preliminary experience of the estimation of cerebral perfusion pressure using transcranial Doppler ultrasonography. J Neurol Neurosurg Psychiatr. 2001;70:198–204.
Varsos GV, Richards H, Kasprowicz M, et al. Critical closing pressure determined with a model of cerebrovascular impedance. J Cereb Blood Flow Metab. 2012;33:235–43.
Nelson RJ, Czosnyka M, Pickard JD, et al. Experimental aspects of cerebrospinal haemo- dynamics: the relationship between blood flow velocity waveform and cerebral autoregulation. J Neurosurg. 1992;31:705–10.
Czosnyka M, Richards HK, Czosnyka Z, Piechnik S, Pickard JD, Chir M. Vascular components of cerebrospinal fluid compensation. J Neurosurg. 1999;90:752–9.
Lopez-Magana JA, Richards HK, Radolovich DK, et al. Critical closing pressure: comparison of three methods. J Cereb Blood Flow Metab. 2009;29(5):987–93.
Gosling RG, King DH. Arterial assessment by Doppler-shift ultrasound. Proc R Soc Med. 1974;67:447–9.
Budohoski K, Schmidt B, Smielewski P, et al. Non-invasively estimated ICP pulse amplitude strongly correlates with outcome after TBI. Acta Neurochir Suppl. 2012;114:121–5.
Schmidt EA, Czosnyka Z, Momjian S, Czosnyka M, Bech RA, Pickard JD. Intracranial baroreflex yielding an early Cushing response in human. Acta Neurochir. 2005;95:253–6.
Wan WH, Ang BT, Wang E. The Cushing response: a case for a review of its role as a physiological reflex. J Clin Neurosci. 2008;15(3):223–8.
Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115(5):654–7.
Miller JD, Becker DP, Ward JD, Sullivan HG, Adams WE, Rosner MJ. Significance of intracranial hypertension in severe head injury. J Neurosurg. 1977;47:503–16.
Rosenberg JB, Shiloh AL, Savel RH, Eisen LA. Non-invasive methods of estimating intracranial pressure. Neurocrit Care. 2011;15(3):599–608.
Rajajee V, Vanaman M, Fletcher JJ, Jacobs TL. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011;15:506–15.
Sekhon MS, Griesdale DE, Robba C, et al. Optic nerve sheath diameter on computed tomography is correlated with simultaneously measured intracranial pressure in patients with severe traumatic brain injury. Intensive Care Med. 2014;40:1267–74.
Reid A, Marchbanks RJ, Bateman DE, Martin AM, Brightwell AP, Pickard JD. Mean Intracranial Pressure monitoring by a non invasive audiological technique: a pilot study. J Neurol Neurosurg Psychiatry. 1989;52:610–2.
Klingelhöfer J, Conrad B, Benecke R, Sander D, Markakis E. Evaluation of intracranial pressure from transcranial Doppler studies in cerebral disease. J Neurol. 1988;235:159–62.
Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007;24(suppl 1):S37–44.
Klingelhöfer J, Conrad B, Benecke R, Sander D, Markakis E. Evaluation of intracranial pressure from transcranial Doppler studies in cerebral disease. J Neurol. 1988;235:159–62.
Ursino M, Giannessi M, Frapparelli M, Magosso E. Effect of cushing response on systemic arterial pressure. IEEE Eng Med Biol Mag. 2009;28(6):63–71.
Barbiro-Michaely E, Mayevsky A. Effects of elevated ICP on brain function: can the multiparametric monitoring system detect the ‘Cushing Response’? Neurol Res. 2003;25(1):42–52.
Acknowledgement
Authors thank Mr. Spencer Harland and Dr. Stefan Piechnik for sharing recorded digital data from experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Marek Czosnyka and Peter Smielewski are co-authors of the ICM+ software (www.neurosurg.cam.ac.uk/icmplus) and receive licensing fees (Cambridge Enterprise Ltd, UK). Chiara Robba, Joseph Donnelly, Mypinder Sekhon, Marcel Aries, Rita Bertuetti, Danilo Cardim declare that they have no conflict of interest.
Ethical Standards
All animal studies have been approved by the appropriate ethics committee and have been performed in accordance of UK Animals Scientific Procedures Act of 1986.
Rights and permissions
About this article
Cite this article
Robba, C., Donnelly, J., Bertuetti, R. et al. Doppler Non-invasive Monitoring of ICP in an Animal Model of Acute Intracranial Hypertension. Neurocrit Care 23, 419–426 (2015). https://doi.org/10.1007/s12028-015-0163-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-015-0163-4