Abstract
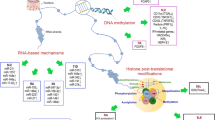
Autoimmune thyroid diseases (AITD), including Graves’ disease and Hashimoto’s thyroiditis, are among the commonest autoimmune disorders, affecting approximately 5 % of the population. Epidemiological data support strong genetic influences on the development of AITD. Since the identification of HLA-DR3 as a major AITD susceptibility gene, there have been significant advances made in our understanding of the genetic mechanisms leading to AITD. We have shown that an amino acid substitution of alanine or glutamine with arginine at position 74 in the HLA-DR peptide binding pocket is a critical factor in the development of AITD, and we are continuing to dissect these mechanisms at the molecular level. In addition to the MHC class II genes, there are now several other confirmed gene loci associated with AITD, including immune-regulatory (CD40, CTLA-4, PTPN22, FOXP3, and CD25) and thyroid-specific genes (thyroglobulin and TSHR). Mechanistically, it is postulated that susceptibility genes interact with certain environmental triggers to induce AITD through epigenetic effects. In this review, we summarize some of the recent advances made in our laboratory dissecting the genetic–epigenetic interactions underlying AITD. As shown in our recent studies, epigenetic modifications offer an attractive mechanistic possibility that can provide further insight into the etiology of AITD.






Similar content being viewed by others
References
Cocks Eschler D, Hasham A, Tomer Y. Cutting edge: the etiology of autoimmune thyroid diseases. Clin Rev Allerg Immunol. 2011;41(2):190–7.
Brent GA. Environmental exposures and autoimmune thyroid disease. Thyroid. 2010;20(7):755–61.
Papanastasiou L, Vatalas IA, Koutras DA, Mastorakos G. Thyroid autoimmunity in the current iodine environment. Thyroid. 2007;17(8):729–39.
Mandac JC, Chaudhry S, Sherman KE, Tomer Y. The clinical and physiological spectrum of interferon-alpha induced thyroiditis: toward a new classification. Hepatology. 2006;43(4):661–72.
Jacobson EM, Huber A, Tomer Y. The HLA gene complex in thyroid autoimmunity: from epidemiology to etiology. J Autoimmun. 2008;30:58–62.
Tomer Y, Huber A. The etiology of autoimmune thyroid disease: a story of genes and environment. J Autoimmun. 2009;32:231–9.
Jacobson EM, Tomer Y. The CD40, CTLA4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: back to the future. J Autoimmun. 2007;28:85–98.
Zamani M, Spaepen M, Bex M, Bouillon R, Cassiman JJ. Primary role of the HLA class II DRB1*0301 allele in graves disease. Am J Med Genet. 2000;95:432–7.
Golden B, Levin L, Ban Y, Concepcion E, Greenberg DA, Tomer Y. Genetic analysis of families with autoimmune diabetes and thyroiditis: evidence for common and unique genes. J Clin Endcrinol Metab. 2005;90(8):4904–11.
Menconi F, Osman R, Monti MC, Greenberg DA, Concepcion ES, Tomer Y. Shared molecular amino acid signature in the HLA-DR peptide binding pocket predisposes to both autoimmune diabetes and thyroiditis. Proc Natl Acad Sci USA. 2010;107(39):16899–903.
Tomer Y. Genetic susceptibility to autoimmune thyroid disease: past, present, and future. Thyroid. 2010;20(7):715–25.
Ban Y, Davies TF, Greenberg DA, Concepcion ES, Osman R, Oashi R, Tomer Y. Arginine at position 74 of the HLA-DR beta1 chain is associated with Graves’ disease. Genes Immun. 2004;5(3):203–8.
Aitman TJ, Todd JA. Molecular genetics of diabetes mellitus. Baillieres Clin Endocrinol Metab. 1995;9:631–56.
Morel PA, Dorman JS, Todd JA, McDevitt HO, Trucco M. Aspartic acid at position 57 of the HLA-DQ beta-chain protects against type 1 diabetes: a family study. Proc Natl Acad Sci USA. 1988;85:8111–5.
Simmonds MJ, Howson JM, Heward JM, Cordell HJ, Foxall H, Carr Smith J, Gibson SM, Walker N, Tomer Y, Franklyn JA, Todd JA, Gough SC. Regression mapping of association between the human leukocyte antigen region and Graves disease. Am J Hum Genet. 2005;76(1):157–63.
Menconi F, Monti MC, Greenberg DA, Oashi T, Osman R, Davies TF, Ban Y, Jacobson EM, Concepcion ES, Li CW, Tomer Y. Molecular amino acid signatures in the MHC class II peptide binding pocket predispose to autoimmune thyroiditis in humans and in mice. Proc Natl Acad Sci USA. 2008;105(37):14034–9.
Hodge SE, Ban Y, Strug LJ, Greenberg DA, Davies TF, Concepcion ES, Villanueva R, Tomer Y. Possible interaction between HLA-DRβ1 and thyroglobulin variants in Graves’ disease. Thyroid. 2006;16:351–5.
Tomer Y, Ban Y, Concepcion E, Barbesino G, Villanueva R, Greenberg DA, Davies TF. Common and unique susceptibility loci in Graves’ and Hashimoto disease: results of whole genome screening in a data set of 102 multiplex families. Am J Hum Genet. 2003;73(4):736–47.
Sakai K, Shirasawa S, Ishikawa N, Ito K, Tamai H, Kuma K, Akamizu T, Tanimura M, Furugaki K, Yamamoto K, Sasazuki T. Identification of susceptibility loci for autoimmune thyroid disease to 5q31-q33 and Hashimoto’s thyroiditis to 8q23-q24 by multipoint affected sib-pair linkage analysis in Japanese. Hum Mol Genet. 2001;10(13):1379–86.
Ban Y, Greenberg DA, Concepcion ES, Skrabanek L, Villanueva R, Tomer Y. Amino acid substitutions in the thyroglobulin gene are associated with susceptibility to human and murine autoimmune thyroid disease. Proc Natl Acad Sci USA. 2003;100:15119–24.
Jacobson EM, Yang H, Menconi F, Wang R, Osman R, Skrabanek L, Li CW, Fadlalla M, Gandhi A, Chaturvedi V, Smith EP, Schwemberger S, Osterburg A, Babcock GF, Tomer Y. Employing a recombinant HLA-DR3 expression system to dissect major histocompatibility complex II-thyroglobulin peptide dynamism: a genetic, biochemical, and reverse immunological perspective. J Biol Chem. 2009;284(49):34231–43.
Stefan M, Jacobson EM, Huber AK, Greenberg DA, Li CW, Skrabanek L, Concepcion E, Fadlalla M, Ho K, Tomer Y. Novel variant of thyroglobulin promoter triggers thyroid autoimmunity through an epigenetic interferon α-modulated mechanism. J Biol Chem. 2011;286(36):31168–79.
Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–55.
Tomer Y, Concepcion E, Greenberg DA. A C/T single nucleotide polymorphism in the region of the CD40 gene is associated with Graves’ disease. Thyroid. 2002;12(12):1129–35.
Ban Y, Tozaki T, Taniyama M, Tomita M, Ban Y. Association of a C/T single nucleotide polymorphism in the 5′ untranslated region of the CD40 gene with Graves’ disease in Japanese. Thyroid. 2006;16(5):443–6.
Jacobson EM, Huber AK, Akeno N, Sivak M, Li CW, Concepcion E, Ho K, Tomer Y. A CD40 Kozak sequence polymorphism and susceptibility to antibody-mediated autoimmune conditions: the role of CD40 tissue-specific expression. Genes Immun. 2007;8(3):205–14.
Jacobson EM, Concepcion E, Oashi T, Tomer Y. A Graves’ disease-associated kozak sequence single-nucleotide polymorphism enhances the efficiency of CD40 gene translation: a case for translational pathophysiology. Endocrinology. 2005;146(6):2684–91.
Sato S, Hasegawa M, Fujimoto M, Tedder TF, Takehara K. Quantitative genetic variation in CD19 expression correlates with autoimmunity. J Immunol. 2000;165:6635–43.
Metcalfe RA, McIntosh RS, Marelli-Berg F, Lombardi G, Lechler R, Weetman AP. Detection of CD40 on human thyroid follicular cells: analysis of expression and function. J Clin Endocrinol Metab. 1998;83(4):1268–74.
Jungel A, Ospelt C, Gay S. What can we learn from epigenetics in the year 2009? Curr Opin Rheumatol. 2010;22(3):284–92.
Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–37.
Bell CG, Teschendorff AE, Rakyan VK, Maxwell AP, Beck S, Savage DA. Genome-wide DNA methylation analysis for diabetic nephropathy in type 1 diabetes mellitus. BMC Med Genomics. 2010;3:33.
Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R711–22.
Karouzakis E, Gay RE, Gay S, Neidhart M. Epigenetic control in rheumatoid arthritis synovial fibroblasts. Nat Rev Rheumatol. 2009;5(5):266–72.
Youngblood B, Reich NO. The early expressed HIV-1 genes regulate DNMT1 expression. Epigenetics. 2008;3(3):149–56.
Acknowledgments
This work was supported in part by National Institutes of Health Grants DK61659, DK067555, and DK073681. This work was also supported by a Veterans Affairs merit award (to Y.T.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hasham, A., Tomer, Y. Genetic and epigenetic mechanisms in thyroid autoimmunity. Immunol Res 54, 204–213 (2012). https://doi.org/10.1007/s12026-012-8302-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-012-8302-x