Abstract
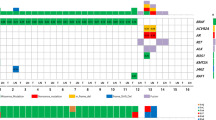
PI3K/Akt signaling pathway plays critical role in many cell processes. There is indication that enhanced activation of PI3K/Akt cascade is implicated in thyroid tumors. Aim of this study was to evaluate the mutational status and expression of PI3K/Akt pathway mediators in papillary thyroid carcinoma in Greece. We evaluated the presence of mutations in PIK3CA (exons 9 and 20), AKT1 (exons 6–11), AKT2 (exons 6–11), AKT3 (exons 5–10), PTEN (exons 3–8), and PDPK1 (exons 4–10) genes in 83 papillary thyroid carcinomas by DNA sequencing. The expression levels of phospho-Akt and insulin-like growth factor I receptor (IGF-IR) were evaluated by immunohistochemistry. PIK3CA mutations were found in three samples. The analysis of AKT1 revealed one silent mutation in exon 9 (G726A) in 16 samples. One specimen carried an AKT3 mutation. One missense mutation was found in one sample in PTEN. No mutations were found in AKT2 and PDPK1. Increased levels of phosphorylated total Akt and IGF-IR were identified in some papillary cancers. Our findings indicate that PI3K/Akt signaling pathway is activated in some papillary tumors. However, mutations in genes coding most mediators of the pathway have not been proven to be the major modus of enhanced activation. These data suggest a potential role for PI3K/Akt-mediated signaling in papillary thyroid tumors.



Similar content being viewed by others
References
Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 141(3):259–77, 1995.
Williams ED, Doniach I, Bjarnason O, Michie W. Thyroid cancer in an iodide rich area: a histopathological study. Cancer 39(1):215–22, 1977.
Harach HR, Ceballos GA. Thyroid cancer, thyroiditis and dietary iodine: a review based on the Salta, Argentina model. Endocr Pathol. 19(4):209–20, 2008.
Imaizumi M, Usa T, Tominaga T, Neriishi K, Akahoshi M, Nakashima E et al. Radiation dose-response relationships for thyroid nodules and autoimmune thyroid diseases in Hiroshima and Nagasaki atomic bomb survivors 55-58 years after radiation exposure. JAMA. 295(9):1011–22, 2006.
Hamatani K, Eguchi H, Ito R, Mukai M, Takahashi K, Taga M et al.. RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res. 68(17):7176–82, 2008.
Rabes HM, Demidchik EP, Sidorow JD, Lengfelder E, Beimfohr C, Hoelzel D et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-Chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin Cancer Res. 6(3):1093–103, 2000.
Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 63(7):1454–7, 2003.
Soares P, Trovisco V, Rocha AS, Lima J, Castro P, Preto A et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 22(29):4578–80, 2003.
Frattini M, Ferrario C, Bressan P, Balestra D, De Cecco L, Mondellini P et al. Alternative mutations of BRAF, RET and NTRK1 are associated with similar but distinct gene expression patterns in papillary thyroid cancer. Oncogene. 23(44):7436-40, 2004.
Leevers SJ, Vanhaesebroeck B, Waterfield MD. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol. 11(2):219–25, 1999. Review.
Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2(7):489–501, 2002.
Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 30(2):193–204, 2004. Review.
Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 21(1):99–102, 1999.
Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 18(1):77–82, 2006.
Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 94(4):455–9, 2006. Review.
Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 4(12):988–1004, 2005. Review.
Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 96(8):4240–5, 1999. Review.
Weng LP, Smith WM, Dahia PL, Ziebold U, Gil E, Lees JA et al. PTEN suppresses breast cancer cell growth by phosphatase activity-dependent G1 arrest followed by cell death. Cancer Res. 59(22):5808–14, 1999.
Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 22(14):2954–63, 2004.
Ringel MD, Hayre N, Saito J, Saunier B, Schuppert F, Burch H et al. Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res. 61(16):6105–11, 2001.
Miyakawa M, Tsushima T, Murakami H, Wakai K, Isozaki O, Takano K. Increased expression of phosphorylated p70S6 kinase and Akt in papillary thyroid cancer tissues. Endocr J. 50(1):77–83, 2003.
Vasko V, Saji M, Hardy E, Kruhlak M, Larin A, Savchenko V et al. Akt activation and localisation correlate with tumour invasion and oncogene expression in thyroid cancer. J Med Genet. 41(3):161–70, 2004.
Dahia PL, Marsh DJ, Zheng Z, Zedenius J, Komminoth P, Frisk T et al. Somatic deletions and mutations in the Cowden disease gene, PTEN, in sporadic thyroid tumors. Cancer Res. 57(21):4710–3, 1997.
Wu G, Mambo E, Guo Z, Hu S, Huang X, Gollin SM et al. Uncommon mutation, but common amplifications, of the PIK3CA gene in thyroid tumors. J Clin Endocrinol Metab. 90(8):4688–93, 2005.
Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 304(5670):554, 2004.
Minaguchi T, Yoshikawa H, Oda K, Ishino T, Yasugi T, Onda T et al. PTEN mutation located only outside exons 5, 6, and 7 is an independent predictor of favorable survival in endometrial carcinomas. Clin Cancer Res. 7(9):2636–42, 2001.
Konopka B, Paszko Z, Janiec-Jankowska A, Goluda M. Assessment of the quality and frequency of mutations occurrence in PTEN gene in endometrial carcinomas and hyperplasias. Cancer Lett. 178(1):43–51, 2002.
Mitsiades CS, Kotoula V, Poulaki V, Sozopoulos E, Negri J, Charalambous E et al. Epidermal growth factor receptor as a therapeutic target in human thyroid carcinoma: mutational and functional analysis. J Clin Endocrinol Metab. 91(9):3662–6, 2006.
Miled N, Yan Y, Hon WC, Perisic O, Zvelebil M, Inbar Y et al. Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic subunit. Science. 317(5835):239–42, 2007.
Hunter C, Smith R, Cahill DP, Stephens P, Stevens C, Teague J et al. A hypermutation phenotype and somatic MSH6 mutations in recurrent human malignant gliomas after alkylator chemotherapy. Cancer Res. 66(8):3987–91, 2006.
Hou P, Liu D, Shan Y, Hu S, Studeman K, Condouris S et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res. 13(4):1161–70, 2007.
Wang Y, Hou P, Yu H, Wang W, Ji M, Zhao S et al. High prevalence and mutual exclusivity of genetic alterations in the phosphatidylinositol-3-kinase/Akt pathway in thyroid tumors. J Clin Endocrinol Metab. 92(6):2387–90, 2007.
Cass LA, Meinkoth JL. Ras signaling through PI3K confers hormone-independent proliferation that is compatible with differentiation. Oncogene. 19(7):924–32, 2000.
Kimura T, Dumont JE, Fusco A, Golstein J. Insulin and TSH promote growth in size of PC Cl3 rat thyroid cells, possibly via a pathway different from DNA synthesis: comparison with FRTL-5 cells. Eur J Endocrinol. 140(1):94–103, 1999.
De Vita G, Berlingieri MT, Visconti R, Castellone MD, Viglietto G, Baldassarre G et al. Akt/protein kinase B promotes survival and hormone-independent proliferation of thyroid cells in the absence of dedifferentiating and transforming effects. Cancer Res. 60(14):3916–20, 2000.
Saito J, Kohn AD, Roth RA, Noguchi Y, Tatsumo I, Hirai A et al. Regulation of FRTL-5 thyroid cell growth by phosphatidylinositol (OH) 3 kinase-dependent Akt-mediated signaling. Thyroid. 11(4):339–51, 2001.
Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 16(1):64–7, 1997.
Ali IU, Schriml LM, Dean M. Mutational spectra of PTEN/MMAC1 gene: a tumor suppressor with lipid phosphatase activity. J Natl Cancer Inst. 91(22):1922–32, 1999.
Marsh DJ, Dahia PL, Zheng Z, Liaw D, Parsons R, Gorlin RJ et al. Germline mutations in PTEN are present in Bannayan–Zonana syndrome. Nat Genet. 16(4):333–4, 1997.
Di Renzo MF, Olivero M, Ferro S, Prat M, Bongarzone I, Pilotti S et al. Overexpression of the c-MET/HGF receptor gene in human thyroid carcinomas. Oncogene. 7(12):2549–53, 1992.
Gire V, Marshall C, Wynford-Thomas D. PI-3-kinase is an essential anti-apoptotic effector in the proliferative response of primary human epithelial cells to mutant RAS. Oncogene. 19(19):2269–76, 2000.
Xing S, Furminger TL, Tong Q, Jhiang SM. Signal transduction pathways activated by RET oncoproteins in PC12 pheochromocytoma cells. J Biol Chem. 273(9):4909–14, 1998.
Matsuo K, Tang SH, Fagin JA. Allelotype of human thyroid tumors: loss of chromosome 11q13 sequences in follicular neoplasms. Mol Endocrinol. 5(12):1873–9, 1991.
De Vita G, Melillo RM, Carlomagno F, Visconti R, Castellone MD, Bellacosa A et al. Tyrosine 1062 of RET-MEN2A mediates activation of Akt (protein kinase B) and mitogen-activated protein kinase pathways leading to PC12 cell survival. Cancer Res. 60(14):3727–31, 2000.
Eccles N, Ivan M, Wynford-Thomas D. Mitogenic stimulation of normal and oncogene-transformed human thyroid epithelial cells by hepatocyte growth factor. Mol Cell Endocrinol. 117(2):247–51, 1996.
Ivan M, Bond JA, Prat M, Comoglio PM, Wynford-Thomas D. Activated ras and ret oncogenes induce over-expression of c-met (hepatocyte growth factor receptor) in human thyroid epithelial cells. Oncogene. 14(20):2417–23, 1997.
Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 370(6490):527–32, 1994.
Espinosa AV, Porchia L, Ringel MD. Targeting BRAF in thyroid cancer. Br J Cancer. 96(1):16–20, 2007. Review.
Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV et al. A "silent" polymorphism in the MDR1 gene changes substrate specificity. Science. 315(5811):525–8, 2007. Erratum in: Science. 2007 Nov 30;318(5855):1382–3.
Komar AA. Silent SNPs: impact on gene function and phenotype. Pharmacogenomics. 8(8):1075–80, 2007. Review.
Sauna ZE, Kimchi-Sarfaty C, Ambudkar SV, Gottesman MM. Silent polymorphisms speak: how they affect pharmacogenomics and the treatment of cancer. Cancer Res. 67(20):9609–12, 2007. Review.
Hunt R, Sauna ZE, Ambudkar SV, Gottesman MM, Kimchi-Sarfaty C. Silent (synonymous) SNPs: should we care about them? Methods Mol Biol. 578:23–39, 2009.
Santarpia L, El-Naggar AK, Cote GJ, Myers JN, Sherman SI. Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J Clin Endocrinol Metab. 93(1):278–84, 2008.
Paes JE, Ringel MD. Dysregulation of the phosphatidylinositol 3-kinase pathway in thyroid neoplasia. Endocrinol Metab Clin North Am. 37(2):375–87, 2008, viii–ix.
Wick MJ, Dong LQ, Riojas RA, Ramos FJ, Liu F. Mechanism of phosphorylation of protein kinase B/Akt by a constitutively active 3-phosphoinositide-dependent protein kinase-1. J Biol Chem. 275(51):40400–6, 2000.
Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 107(6):873–7, 2003.
Baserga R, Resnicoff M, Dews M. The IGF-I receptor and cancer. Endocrine. 7(1):99–102, 1997. Review. No abstract available.
Bergmann U, Funatomi H, Yokoyama M, Beger HG, Korc M. Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Res. 55(10):2007–11, 1995.
Gydee H, O'Neill JT, Patel A, Bauer AJ, Tuttle RM, Francis GL. Differentiated thyroid carcinomas from children and adolescents express IGF-I and the IGF-I receptor (IGF-I-R). Cancers with the most intense IGF-I-R expression may be more aggressive. Pediatr Res. 55(4):709–15, 2004.
Maiorano E, Ciampolillo A, Viale G, Maisonneuve P, Ambrosi A, Triggiani V et al. Insulin-like growth factor 1 expression in thyroid tumors. Appl Immunohistochem Mol Morphol. 8(2):110–9, 2000.
Yashiro T, Ohba Y, Murakami H, Obara T, Tsushima T, Fujimoto Y et al. Expression of insulin-like growth factor receptors in primary human thyroid neoplasms. Acta Endocrinol (Copenh). 121(1):112–20, 1989.
Kornprat P, Rehak P, Rüschoff J, Langner C. Expression of IGF-I, IGF-II, and IGF-IR in gallbladder carcinoma. A systematic analysis including primary and corresponding metastatic tumours. J Clin Pathol. 59(2):202–6, 2006.
Parker AS, Cheville JC, Janney CA, Cerhan JR. High expression levels of insulin-like growth factor-I receptor predict poor survival among women with clear-cell renal cell carcinomas. Hum Pathol. 33(8):801–5, 2002.
Schillaci R, Galeano A, Becu-Villalobos D, Spinelli O, Sapia S, Bezares RF. Autocrine/paracrine involvement of insulin-like growth factor-I and its receptor in chronic lymphocytic leukaemia. Br J Haematol. 130(1):58–66, 2005.
Vella V, Pandini G, Sciacca L, Mineo R, Vigneri R, Pezzino V et al. A novel autocrine loop involving IGF-II and the insulin receptor isoform-A stimulates growth of thyroid cancer. J Clin Endocrinol Metab. 87(1):245–54, 2002.
Chakravarty G, Santillan AA, Galer C, Adams HP, El-Naggar AK, Jasser SA et al. Phosphorylated insulin like growth factor-I receptor expression and its clinico-pathological significance in histologic subtypes of human thyroid cancer. Exp Biol Med (Maywood). 234(4):372–86, 2009.
Acknowledgments
This study is part of the 03ED375 research project, implemented within the framework of (PENED) and cofinanced by National and Community Funds (EU—European Social Fund and Greek Ministry of Development, General Secretariat of Research and Technology). The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sozopoulos, E., Litsiou, H., Voutsinas, G. et al. Mutational and Immunohistochemical Study of the PI3K/Akt Pathway in Papillary Thyroid Carcinoma in Greece. Endocr Pathol 21, 90–100 (2010). https://doi.org/10.1007/s12022-010-9112-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-010-9112-0