Abstract
Purpose
In this study, we investigated the profile of microRNAs (miRNAs) contained in exosomes secreted in the serum of patients with papillary thyroid cancer (PTC).
Methods
Exosome were isolated by adding ExoQuick Exosome Precipitation Solution. Dynamic light scattering (DLS) and western blotting analysis were used to ensure the quality of exosomes. The expression levels of miRNAs were investigated using custom-designed TaqMan Advanced miRNA Array Cards in the screening cohort and using specific TaqMan Advanced MicroRNA Assays in the validation cohort.
Results
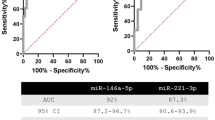
We identified miR24-3p, miR146a-5p, miR181a-5p and miR382-5p with different expression levels in two different series of 56 and 58 PTC patients as compared with healthy controls. Significant differences in the expression of three PTC exosomal miRNAs, depending on the presence of lymph node metastasis, were detected in only one PTC series. When comparing the expression levels of some PTC-specific exosomal miRNAs with those of the same miRNAs circulating free of any encapsulation, we found a significant correlation for only miR24-3p, suggesting that only select miRNAs are secreted in exosomes.
Conclusions
Our findings demonstrate that four miRNAs are differently secreted in the exosomes of PTC patients, whereas no conclusive results were found to characterize PTCs with lymph node metastasis, suggesting caution in the use of circulating exosomal miRNA expression levels as lymph node metastasis biomarkers. Further investigation into the mechanisms governing miRNA secretion in tumor cells are required.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
M.E. Cabanillas, D.G. McFadden, C. Durante, Thyroid cancer. Lancet 388(10061), 2783–2795 (2016)
A. Zomer, J. van Rheenen, Implications of extracellular vesicle transfer on cellular heterogeneity in cancer: what are the potential clinical ramifications? Cancer Res. 76(8), 2071–2075 (2016)
W. Guo, Y. Gao, N. Li, F. Shao, C. Wang, P. Wang, Z. Yang, R. Li, J. He, Exosomes: new players in cancer. Oncol. Rep. 38, 665–675 (2017)
C.F. Ruivo, B. Adem, M. Silva, S.A. Melo, The biology of cancer exosomes: insights and new perspectives. Cancer Res. 77, 6480–6488 (2017)
M.P. Bebelman, M.J. Smit, D.M. Pegtel, S.R. Baglio, Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 188, 1–11 (2018)
K. Agarwal, M. Saji, S.M. Lazaroff, A.F. Palmer, M.D. Ringel, M.E. Paulaitis, Analysis of exosome release as a cellular response to MAPK pathway inhibition. Langmuir 31(19), 5440–5448 (2015)
M. Xing, Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 13(3), 184–199 (2013)
A. Prete, P. Borges de Souza, S. Censi, M. Muzza, N. Nucci, M. Sponziello, Update on fundamental mechanisms of thyroid cancer. Front. Endocrinol. 11, 102 (2020)
S. Subramaniam, V. Jeet, J.A. Clements, J.H. Gunter, J. Batra, Emergence of microRNAs as key players in cancer cell metabolism. Clin. Chem. 65(9), 1090–1101 (2019)
K. Babaei, S. Shams, A. Keymoradzadeh, S. Vahidi, P. Hamami, R. Khaksar, S.E. Norollahi, A.A. Samadani, An insight of microRNAs performance in carcinogenesis and tumorigenesis; an overview of cancer therapy. Life Sci. 240, 117077 (2020)
A.Z. Syeda, S.S.S. Langden, C. Munkhzul, M. Lee, S.J. Song, Regulatory mechanism of microRNA expression in cancer. Int. J. Mol. Sci. 21(5), 1723 (2020)
M. Celano, F. Rosignolo, V. Maggisano, V. Pecce, M. Iannone, D. Russo, S. Bulotta, MicroRNAs as biomarkers in thyroid carcinoma. Int. J. Genom. 2017, 6496570 (2017)
M. Swierniak, A. Wojcicka, M. Czetwertynska, E. Stachlewska, M. Maciag, W. Wiechno, B. Gornicka, M. Bogdanska, L. Koperski, A. de la Chapelle, K. Jazdzewski, In-depth characterization of the microRNA transcriptome in normal thyroid and papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 98(8), E1401–E1409 (2013)
Y. Peng, C. Li, D.C. Luo, J.W. Ding, W. Zhang, G. Pan, Expression profile and clinical significance of microRNAs in papillary thyroid carcinoma. Molecules 19(8), 11586–11599 (2014)
F. Rosignolo, M. Sponziello, L. Giacomelli, D. Russo, V. Pecce, M. Biffoni, R. Bellantone, C.P. Lombardi, L. Lamartina, G. Grani, C. Durante, S. Filetti, A. Verrienti, Identification of thyroid-associated serum microRNA profiles and their potential use in thyroid cancer follow-up. J. Endocr. Soc. 1(1), 3–13 (2017)
F. Rosignolo, L. Memeo, F. Monzani, C. Colarossi, V. Pecce, A. Verrienti, C. Durante, G. Grani, L. Lamartina, S. Forte, D. Martinetti, D. Giuffrida, D. Russo, F. Basolo, S. Filetti, M. Sponziello, MicroRNA-based molecular classification of papillary thyroid carcinoma. Int. J. Oncol. 50(5), 1767–1777 (2017)
D. Dai, Y. Tan, L. Guo, A. Tang, Y. Zhao, Identification of exosomal miRNA biomarkers for diagnosis of papillary thyroid cancer by small RNA sequencing. Eur. J. Endocrinol. 182(1), 111–121 (2020)
A. Gagliardi, S. Voci, M.C. Salvatici, M. Fresta, D. Cosco, Brij-stabilized zein nanoparticles as potential drug carriers. Colloids Surf. B. Biointerfaces 201, 111647 (2021)
V. Pecce, M. Sponziello, G. Damante, F. Rosignolo, C. Durante, L. Lamartina, G. Grani, D. Russo, C.R. Di Gioia, S. Filetti, A. Verrienti, A synonymous RET substitution enhances the oncogenic effect of an in-cis missense mutation by increasing constitutive splicing efficiency. PLoS Genet. 14(10), e1007678 (2018)
V. Maggisano, M. Celano, S.M. Lepore, M. Sponziello, F. Rosignolo, V. Pecce, A. Verrienti, F. Baldan, C. Mio, L. Allegri, M. Maranghi, R. Falcone, G. Damante, D. Russo, S. Bulotta, Human telomerase reverse transcriptase in papillary thyroid cancer: gene expression, effects of silencing and regulation by BET inhibitors in thyroid cancer cells. Endocrine 63, 545–553 (2019)
V. Maggisano, M. Celano, G.E. Lombardo, S.M. Lepore, M. Sponziello, F. Rosignolo, A. Verrienti, F. Baldan, E. Puxeddu, C. Durante, S. Filetti, G. Damante, D. Russo, S. Bulotta, Silencing of hTERT blocks growth and migration of anaplastic thyroid cancer cells. Mol. Cell. Endocrinol. 448, 34–40 (2017)
The Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 159(3), 676–690 (2014)
R. Samsonov, V. Burdakov, T. Shtam, Z. Radzhabovа, D. Vasilyev, E. Tsyrlina, S. Titov, M. Ivanov, L. Berstein, M. Filatov, N. Kolesnikov, H. Gil-Henn, A. Malek, Plasma exosomal miR-21 and miR-181a differentiates follicular from papillary thyroid cancer. Tumor Biol. 37(9), 12011–12021 (2016)
J.C. Lee, J.T. Zhao, J. Gundara, J. Serpell, L.A. Bach, S. Sidhu, Papillary thyroid cancer-derived exosomes contain miRNA-146b and miRNA-222. J. Surg. Res. 196(1), 39–48 (2015)
K. Jiang, G. Li, W. Chen, L. Song, T. Wei, Z. Li, R. Gong, J. Lei, H. Shi, J. Zhu, Plasma exosomal miR-146b-5p and miR-222-3p are potential biomarkers for lymph node metastasis in papillary thyroid carcinomas. OncoTargets Ther. 13, 1311–1319 (2020)
Q. Pan, Z.J. Jiangman, M. Li, X. Liu, Y. Xu, W. Li, S. Wu, Z. Su, Exosomal miRNAs are potential diagnostic biomarkers between malignant and benign thyroid nodules based on next-generation sequencing. Carcinogenesis 41(1), 18–24 (2020)
F. Wu, F. Li, X. Lin, F. Xu, R.R. Cui, J.Y. Zhong, T. Zhu, S.K. Shan, X.B. Liao, L.Q. Yuan, Z.H. Mo, Exosomes increased angiogenesis in papillary thyroid cancer microenvironment. Endocr. Relat. Cancer 26(5), 525–538 (2019)
Y.W. Yi, J.H. Lee, S. Kim, C. Pack, D.H. Ha, S.R. Park, J. Youn, B.S. Cho, Advances in analysis of biodistribution of exosomes by molecular imaging. Int. J. Mol. Sci. 21(2), 665 (2020)
S. Bulotta, M. Celano, G. Costante, D. Russo, Novel therapeutic options for radioiodine-refractory thyroid cancer: redifferentiation and beyond. Curr. Opin. Oncol. 32(1), 13–19 (2020)
L. Lamartina, G. Grani, M. Biffoni, L. Giacomelli, G. Costante, S. Lupo, M. Maranghi, K. Plasmati, M. Sponziello, F. Trulli, A. Verrienti, S. Filetti, C. Durante, Risk stratification of neck lesions detected sonographically during the follow-up of differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 101(8), 3036–3044 (2016)
G. Pogliaghi, Liquid biopsy in thyroid cancer: from circulating biomarkers to a new prospective of tumor monitoring and therapy. Minerva Endocrinol. 46(1), 45–61 (2021). https://doi.org/10.23736/S2724-6507.20.03339-8
Y. Zhang, D. Xu, J. Pan, Z. Yang, M. Chen, J. Han, S. Zhang, L. Sun, H. Qiao, Dynamic monitoring of circulating microRNAs as a predictive biomarker for the diagnosis and recurrence of papillary thyroid carcinoma. Oncol. Lett. 13(6), 4252–4266 (2017)
J.C. Lee, J.T. Zhao, R.J. Clifton-Bligh, A. Gill, J.S. Gundara, C. Ip, J. Glover, A. Sywak, M.S. Delbridge, L.W. Robinson, B.G. Sidhu, S.B.: MicroRNA-222 and microRNA-146b are tissue and circulating biomarkers of recurrent papillary thyroid cancer. Cancer 119(24), 4358–4365 (2013)
R. Benedetti, C. Papulino, G. Sgueglia, U. Chianese, T. De Marchi, F. Iovino, D. Rotili, A. Mai, E. Niméus, C. Dell’Aversana, L. Altucci, Regulatory interplay between miR-181a-5p and estrogen receptor signaling cascade in breast cancer. Cancers 13(3), 543 (2021)
T. Bjørnetrø, K.R. Redalen, S. Meltzer, N.S. Thusyanthan, R. Samiappan, C. Jegerschöld, K.R. Handeland, A.H. Ree, An experimental strategy unveiling exosomal microRNAs 486-5p, 181a-5p and 30d-5p from hypoxic tumour cells as circulating indicators of high-risk rectal Cancer. J. Extracell. Vesicles. 8(1), 1567219 (2019)
J.R. Jacona, C.S. Lutz, miR-146a-5p: expression, regulation, and functions in cancer. Wiley Interdiscip. Rev. RNA 10(4), e1533 (2019)
C. Yin, Q. Han, D. Xu, B. Zheng, X. Zhao, J. Zhang, SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. Oncoimmunology 8(7), 1601479 (2019)
D.-L. Yuwen, B.-B. Sheng, J. Liu, W. Wenyu, Y.-Q. Shu, MiR-146a-5p level in serum exosomes predicts therapeutic effect of cisplatin in non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 21(11), 2650–2658 (2017)
Y. Zhang, J. Zhao, M. Ding, Y. Su, D. Cui, C. Jiang, S. Zhao, G. Jia, X. Wang, Y. Ruan, Y. Jing, S. Xia, B. Han, Loss of exosomal miR-146a-5p from cancerassociated fibroblasts after androgen deprivation therapy contributes to prostate cancer metastasis. J. Exp. Clin. Cancer Res. 39(1), 282 (2020)
Q. Wu, L. Yu, X. Lin, Q. Zheng, S. Zhang, D. Chen, X. Pan, Y. Huang, Combination of serum miRNAs with serum exosomal miRNAs in early diagnosis for non-small-cell lung cancer. Cancer Manag. Res. 12, 485–495 (2020)
J. Wang, C. Chen, X. Yan, P. Wang, The role of miR-382-5p in glioma cell proliferation, migration and invasion. Onco Targets Ther. 12, 4993–5002 (2019)
I.H. Lee, G. Kim, S.G. Kwak, D.W. Baek, B.W. Kang, H.J. Kim, S.Y. Park, J.S. Park, G.S. Choi, K. Hur, J.G. Kim, Predictive value of circulating miRNAs in lymph node metastasis for colon cancer. Genes. 12(2), 176 (2021)
H. Wang, C. Chen, K. Ding, W. Zhang, J. Hou, MiR-24-3p as a prognostic indicator for multiple cancers: from a meta-analysis view. Biosci. Rep. 40(12), BSR20202938 (2020)
M. Liang, F. Zhan, J. Zhao, Q. Li, J. Wuyang, G. Mu, D. Li, Y. Zhang, X. Huang, A panel of plasma exosomal miRNAs as potential biomarkers for differential diagnosis of thyroid nodules. Front. Genet. 11, 449 (2020).
B.R. Haugen, E.K. Alexander, K.C. Bible, G. Doherty, S.J. Mandel, Y.E. Nikiforov, F. Pacini, G. Randolph, A. Sawka, M. Schlumberger, K.G. Schuff, S.I. Sherman, J.A. Sosa, D. Steward, R.M. Tuttle, L. Wartofsky, 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid 26, 1–133 (2016)
Acknowledgements
The manuscript was edited by Melissa Kerr.
Funding
This research was funded by PRIN2017EKMFTN_003.
Author information
Authors and Affiliations
Contributions
Conceptualization, F.C., A.V. and D.R.; validation, V.P. and M.S.; meth-odology, L.G. and V.A.; formal analysis, V.M., and A.G.; investigation, F.C., V.M., A.V. and M.C.; writing—original draft preparation, S.B., F.C. and A.V.; writing—review and editing, D.R., S.B.; supervision, D.R., C.D. and S.B. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This study was conducted according to guidelines of the Declaration of Helsinki and approved by the ethics committee of Sapienza University of Rome (protocol code 1184/17 and date of approval 21/12/2017).
Consent for publication
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Capriglione, F., Verrienti, A., Celano, M. et al. Analysis of serum microRNA in exosomal vehicles of papillary thyroid cancer. Endocrine 75, 185–193 (2022). https://doi.org/10.1007/s12020-021-02847-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02847-2