Abstract
Purpose
Glucagon stimulation test (GST) is used to assess the hypothalamo-pituitary-adrenal (HPA) and growth hormone (GH) axes with an incompletely defined mechanism. We aimed to assess if glucagon acted through fibroblast growth factor-21 (FGF-21) to stimulate cortisol and GH secretion. The secondary outcome was to determine the relationship of FGF-21 with variable GH responses to GST in obesity.
Methods:
A total of 26 healthy participants; 11 obese (body mass index (BMI) > 30 kg/m2) and 15 leans (BMI < 25 kg/m2) were included. Basal pituitary and target hormone levels were measured and GST was performed. During GST, glucose, insulin, cortisol, GH, and FGF-21 responses were measured.
Results:
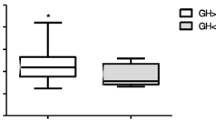
The mean age of the participants was 26.3±3.6 years. Glucagon resulted in significant increases in FGF-21, glucose, insulin, cortisol, and GH levels. The levels of basal cortisol, GH, FGF-21, and IGF-1 were similar in the two groups. The peak GH and area under the curve (AUC)(GH) responses to GST in the obese group were lower than those of the normal-weight group with a different pattern of response. There were no differences between the groups in terms of peak cortisol, AUC(cortisol), peak insulin, AUC(insulin), peak FGF-21, and AUC(FGF21). Obesity was associated with significantly increased glucose and insulin responses and slightly decreased FGF-21 response to glucagon.
Conclusion
Obesity was associated with blunted and delayed GH, but preserved cortisol responses to GST. This is the first study showing that glucagon stimulates the HPA and GH axis independently from FGF-21. The delayed GH response to GST in obesity does not seem to be related to FGF-21.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
K.M. Habegger et al. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes. 62(5), 1453–1463 (2013). https://doi.org/10.2337/db12-1116.
Z. Karaca, A. Grossman, F. Kelestimur Investigation of the Hypothalamo-pituitary-adrenal (HPA) axis: a contemporary synthesis. Rev. Endocr. Metab. Disord. 22(2), 179–204 (2021). https://doi.org/10.1007/s11154-020-09611-3.
E. Arvat et al. Glucagon is an ACTH secretagogue as effective as hCRH after intramuscolar administration while it is ineffective when given intravenously in normal subjects. Pituitary. 3(3), 169–173 (2000). https://doi.org/10.1023/a:1011451710004.
E. Arvat et al. Interaction between glucagon and human corticotropin-releasing hormone or vasopressin on ACTH and cortisol secretion in humans. Eur. J. Endocrinol. 143(1), 99–104 (2000). https://doi.org/10.1530/eje.0.1430099.
F. Broglio et al. Ghrelin does not mediate the somatotroph and corticotroph responses to the stimulatory effect of glucagon or insulin-induced hypoglycaemia in humans. Clin. Endocrinol. (Oxf). 60(6), 699–704 (2004). https://doi.org/10.1111/j.1365-2265.2004.02038.x.
N. Babaknejad, H. Nayeri, R. Hemmati, S. Bahrami, A. Esmaillzadeh An overview of FGF19 and FGF21: the therapeutic role in the treatment of the metabolic disorders and obesity. Horm. Metab. Res. 50(6), 441–452 (2018). https://doi.org/10.1055/a-0623-2909.
Y.H. Youm, T.L. Horvath, D.J. Mangelsdorf, S.A. Kliewer, V.D. Dixit Prolongevity hormone FGF21 protects against immune senescence by delaying age-related thymic involution. Proc. Natl. Acad. Sci. USA. 113(4), 1026–1031 (2016). https://doi.org/10.1073/pnas.1514511113.
Q. Liang et al. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes. 63(12), 4064–4075 (2014). https://doi.org/10.2337/db14-0541.
A. Bartke, L.Y. Sun, V. Longo Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol. Rev. 93(2), 571–598 (2013). https://doi.org/10.1152/physrev.00006.2012.
H.A. Cyphert, K.M. Alonge, S.M. Ippagunta, F.B. Hillgartner Glucagon stimulates hepatic FGF21 secretion through a PKA- and EPAC-dependent posttranscriptional mechanism. PLoS One. 9(4), e94996 (2014). https://doi.org/10.1371/journal.pone.0094996.
A. Salminen, K. Kaarniranta, A. Kauppinen Regulation of longevity by FGF21: interaction between energy metabolism and stress responses. Ageing. Res. Rev. 37, 79–93 (2017). https://doi.org/10.1016/j.arr.2017.05.004.
J.S. Hansen et al. Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans. Mol. Metab. 4(8), 551–560 (2015). https://doi.org/10.1016/j.molmet.2015.06.001.
O. Al-Massadi, J. Ferno, C. Dieguez, R. Nogueiras, M. Quinones Glucagon control on food intake and energy balance. Int. J. Mol. Sci. 20(16), 2019. https://doi.org/10.3390/ijms20163905.
K.S. Leong, A.B. Walker, I. Martin, D. Wile, J. Wilding, I.A. MacFarlaneAn audit of 500 subcutaneous glucagon stimulation tests to assess growth hormone and ACTH secretion in patients with hypothalamic-pituitary disease. Clin. Endocrinol. (Oxf). 54(4), 463–468 (2001). https://doi.org/10.1046/j.1365-2265.2001.01169.x.
H. Makimura, T. Stanley, D. Mun, S.M. You, S. Grinspoon The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J. Clin. Endocrinol. Metab. 93(11), 4254–4260 (2008). https://doi.org/10.1210/jc.2008-1333.
A.L. Bookout et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 19(9), 1147–1152 (2013). https://doi.org/10.1038/nm.3249.
R. Patel et al. Glucocorticoids regulate the metabolic hormone FGF21 in a feed-forward loop. Mol. Endocrinol. 29(2), 213–223 (2015). https://doi.org/10.1210/me.2014-1259.
M. Mraz et al. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin. Endocrinol. (Oxf). 71(3), 369–375 (2009).
T. Jezdimirovic, V. Stajer, V. S. Ostojic, Cardiovascular autonomic reflex tests and serum FGF21 levels in overweight and normal-weight men and women. Arch. Physiol. Biochem 5, 1–5 (2019). https://doi.org/10.1080/13813455.2019.1683586.
C. Giannini et al. Circulating levels of FGF-21 in obese youth: associations with liver fat content and markers of liver damage. J. Clin. Endocrinol. Metab. 98(7), 2993–3000 (2013). https://doi.org/10.1210/jc.2013-1250.
H. Yan et al. Circulating fibroblast growth factor 21 levels are closely associated with hepatic fat content: a cross-sectional study. PLoS One. 6(9), e24895 (2011). https://doi.org/10.1371/journal.pone.0024895.
A.O. Chavez, M. Molina-Carrion, M.A. Abdul-Ghani, F. Folli, R.A. Defronzo, D. Tripathy Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes. Care. 32(8), 1542–1546 (2009). https://doi.org/10.2337/dc09-0684.
A.M. Arafat et al. Glucagon increases circulating fibroblast growth factor 21 independently of endogenous insulin levels: a novel mechanism of glucagon-stimulated lipolysis? Diabetologia. 56(3), 588–597 (2013). https://doi.org/10.1007/s00125-012-2803-y.
B. Emanuelli et al. Interplay between FGF21 and insulin action in the liver regulates metabolism. J. Clin. Invest. 124(2), 515–527 (2014). https://doi.org/10.1172/JCI67353.
M. Keuper, H.U. Haring, H. Staiger Circulating FGF21 levels in human health and metabolic disease. Exp. Clin. Endocrinol. Diabetes. 128(11), 752–770 (2020). https://doi.org/10.1055/a-0879-2968.
J. Lundberg et al. Influence of growth hormone on circulating fibroblast growth factor 21 levels in humans. J Intern. Med. 274(3), 227–232 (2013). https://doi.org/10.1111/joim.12112.
M.J. Potthoff et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl. Acad. Sci. USA. 106(26), 10853–10858 (2009). https://doi.org/10.1073/pnas.0904187106.
T. Inagaki, V.Y. Lin, R. Goetz, M. Mohammadi, D.J. Mangelsdorf, S.A. Kliewer Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 8(1), 77–83 (2008). https://doi.org/10.1016/j.cmet.2008.05.006.
Funding
This study was funded by the Scientific Research Unit of the Erciyes University, under the project number TTU-2019-9216.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Erciyes University Medical Faculty (2019 / 263).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akkar, I., Karaca, Z., Taheri, S. et al. The stimulatory effects of glucagon on cortisol and GH secretion occur independently from FGF-21. Endocrine 75, 211–218 (2022). https://doi.org/10.1007/s12020-021-02829-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02829-4