Abstract
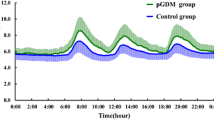
Maternal hyperglycemia in gestational diabetes mellitus (GDM), especially hyperglycemic excursions, is associated with increased risks of adverse pregnancy outcomes. Continuous glucose monitoring (CGM) system (CGMS) is better than intermittent self-measurements in detecting detailed glucose profiles on the magnitude and duration of glucose fluctuations. Hyperglycemia resulted from impaired β cell function. This study analyzed the characteristics of glycemic variability in GDM with 24–28 gestational weeks and its association with β cell function. Thirty GDM with 24–28 gestational weeks (GDM group) were included in this study, and 20 normal gestational women (NGW group) and 20 normal glucose regulation non-pregnant women (NGRW group) were set as controls. The three groups were monitored using the CGMS for consecutive 72 h. The parameters of glycemic variability included the standard deviation of blood glucose (SDBG), mean of continuous 24-h blood glucose (MBG), mean amplitude of glycemic excursions (MAGEs), and mean of daily differences (MODDs). Homeostasis model assessments were applied to access the insulin resistance (HOMA-IR). The early insulinogenic index (ΔI30/ΔG30) and the area under the curve of insulin (AUCI180) derived from 75-g oral glucose tolerance test were applied to evaluate the early-phase insulin secretion and second-phase insulin secretion, respectively. After CGM, MAGE and MBG value increased progressively from NGRW, NGW to GDM group (p < 0.05); MODD and SDBG values of GDM group were all higher than those of NGRW and NGW groups (p < 0.05), but there are no differences in MODD and SDBG between NGRW and NGW groups (p > 0.05). After comparison of β cell function, ΔI30/ΔG30 decreased progressively from NGRW, NGW to GDM group (p < 0.05); HOMA-IR and AUCI180 increased progressively from NGRW, NGW to GDM group (p < 0.05). MAGE value was correlated with ΔI30/ΔG30 and HOMA-IR in GDM group (r = −0.78 and 0.65, respectively, p < 0.05). Multiple linear stepwise regression analysis showed that ΔI30/ΔG30 and HOMA-IR were the independent factors of MAGE (β = −0.61, 0.34, respectively, p < 0.05). Glycemic variability in GDM was higher than in normal pregnant women, and glycemic variability evaluated by MAGE correlates well with impaired early-phase insulin secretion in GDM. Further large-scale studies are needed to formulate treatment strategies to make up for the impaired early-phase insulin secretion and flat glycemic variability, and analyze the association between pregnancy outcomes improvement and glycemic variability remission in GDM.



Similar content being viewed by others
References
M.G. Dalfrà, G. Sartore, G. Di Cianni, G. Mello, C. Lencioni, S. Ottanelli, J. Sposato, F. Valgimigli, C. Scuffi, M. Scalese, A. Lapolla, Glucose variability in diabetic pregnancy. Diabetes Technol. Ther. 13, 853–859 (2011)
K. Cyganek, A. Hebda-Szydlo, J. Skupien, B. Katra, I. Janas, A. Borodako, I. Kaim, T. Klupa, A. Reron, M.T. Malecki, Glycemic control and pregnancy outcomes in women with type 2 diabetes from Poland. The impact of pregnancy planning and a comparison with type 1 diabetes subjects. Endocrine 40, 243–249 (2011)
L. Herranz, L.F. Pallardo, N. Hillman, P. Martin-Vaquero, A. Villarroel, A. Fernandez, Maternal third trimester hyperglycaemic excursions predict large-for-gestational-age infants in type 1 diabetic pregnancy. Diabetes Res. Clin. Pract. 75, 42–46 (2007)
K.K. Kestilä, U.U. Ekblad, T. Rönnemaa, Continuous glucose monitoring versus self-monitoring of blood glucose in the treatment of gestational diabetes mellitus. Diabetes Res. Clin. Pract. 77, 174–179 (2007)
C. Homko, E. Sivan, X. Chen, E.A. Reece, G. Boden, Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 86, 568–573 (2001)
A. Kautzky-Willer, R. Prager, W. Waldhausl, G. Pacini, K. Thomaseth, O.F. Wagner, M. Ulm, C. Streli, B. Ludvik, Pronounced insulin resistance and inadequate beta-cell secretion characterize lean gestational diabetes during and after pregnancy. Diabetes Care 20, 1717–1723 (1997)
American Diabetes Association, Diagnosis and classification of diabetes mellitus. Diabetes Care 31, S55–S60 (2008)
A. Lapolla, M.G. Dalfrà, D. Fedele, Management of gestational diabetes mellitus. Diabetes Metab. Syndr. Obes. 2, 73–82 (2009)
American Diabetes Association, J.P. Bantle, J. Wylie-Rosett, A.L. Albright, C.M. Apovian, N.G. Clark, M.J. Franz, B.J. Hoogwerf, A.H. Lichtenstein, E. Mayer-Davis, A.D. Mooradian, M.L. Wheeler, Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 31, S61–S78 (2008)
J. Zhou, W. Jia, Y. Bao, X. Ma, W. Lu, H. Li, C. Hu, K. Xiang, Glycemic variability and its responses to intensive insulin treatment in newly diagnosed type 2 diabetes. Med. Sci. Monit. 14, CR552–CR558 (2008)
A.L. McCall, D.J. Cox, J. Crean, M. Gloster, B.P. Kovatchev, A novel analytical method for assessing glucose variability: using CGMS in type 1 diabetes mellitus. Diabetes Technol. Ther. 8, 644–653 (2006)
L. Monnier, E. Mas, C. Ginet, F. Michel, L. Villon, J.P. Cristol, C. Colette, Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 295, 1681–1687 (2006)
Y. Song, J.E. Manson, L. Tinker, B.V. Howard, L.H. Kuller, L. Nathan, N. Rifai, S. Liu, Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: the Women’s Health Initiative Observational Study. Diabetes Care 30, 1747–1752 (2007)
C. Pang, Y.Q. Bao, C. Wang, J.X. Lu, W.P. Jia, K.S. Xiang, Relationship between the level of fasting plasma glucose and beta cell functions in Chinese with or without diabetes. Chin. Med. J. (Engl.) 121, 2119–2123 (2008)
R. Oka, K. Yagi, M. Sakurai, K. Nakamura, T. Moriuchi, S. Miyamoto, A. Nohara, M.A. Kawashiri, Y. Takeda, M. Yamagishi, Insulin secretion and insulin sensitivity on the oral glucose tolerance test (OGTT) in middle-aged Japanese. Endocr. J. 59, 55–64 (2012)
H.R. Murphy, G. Rayman, K. Lewis, S. Kelly, B. Johal, K. Duffield, D. Fowler, P.J. Campbell, R.C. Temple, Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ 337, a1680 (2008)
M. Brownlee, Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820 (2001)
M. Brownlee, The pathophysiology of diabetic complications: a unifying mechanism. Diabetes 54, 1615–1625 (2005)
C. Kühl, Etiology and pathogenesis of gestational diabetes. Diabetes Care 21, B19–B26 (1998)
S. Del Prato, P. Marchetti, R.C. Bonadonna, Phasic insulin release and metabolic regulation in type 2 diabetes. Diabetes 51, S109–S116 (2002)
S. Del Prato, Loss of early insulin secretion leads to postprandial hyperglycaemia. Diabetologia 46, M2–M8 (2003)
M.R. Clements, J. Tits, B.T. Kinsley, J. Råstam, H.H. Friberg, R.J. Ligthelm, Improved glycaemic control of thrice-daily biphasic insulin aspart compared with twice-daily biphasic human insulin; a randomized, open-label trial in patients with type 1 or type 2 diabetes. Diabetes Obes. Metab. 10, 229–237 (2008)
V. Balaji, M.S. Balaji, C. Alexander, S. Ashalata, R. Sheela Suganthi, S. Suresh, V. Seshiah, Premixed insulin aspart 30 (Biasp 30) vs. premixed human insulin 30 (BHI 30) in gestational diabetes mellitus—a pilot study. J. Assoc. Physicians India 58, 99–101 (2010)
Acknowledgments
The study was partly funded by the Natural Science Research Program of Nantong University (No. 2010Z82).
Conflict of interest
The authors have no competing interests to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Su, Jb., Wang, Xq., Chen, Jf. et al. Glycemic variability in gestational diabetes mellitus and its association with β cell function. Endocrine 43, 370–375 (2013). https://doi.org/10.1007/s12020-012-9753-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9753-5