Abstract
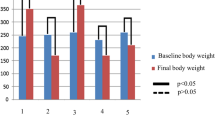
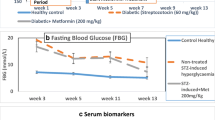
Previous studies have observed changes in the lacrimal gland and ocular surface related to diabetes mellitus and related it to insulin resistance or insufficiency and oxidative damage. The aim of this study was to evaluate whether insulin treatment inhibits those changes. Diabetes was induced in male Wistar rats with a single intravenous injection of streptozotocin and a subgroup was treated with insulin. After 5 and 10 weeks, the three groups (n = 5–10/group/experimental procedure) were compared for biochemical, functional, and histological parameters. After 5 weeks, changes in morphology and increased numbers of lipofucsin-like inclusions were observed in lacrimal glands of diabetic but not insulin-treated rats. After 5 weeks, malonaldehyde and total peroxidase activity were significantly higher in diabetic rats, but similar to control in insulin-treated diabetic rats (P = 0.03, P = 0.02, respectively). Our data indicate that diabetes induces histological alterations in lacrimal gland and suggests that hyperglycemia-related oxidative stress may participate in diabetic dry eye syndrome. Prevention by insulin replacement suggests direct hormone action and/or benefit by early sub optimal metabolic control.





Similar content being viewed by others
References
M. Alves, V.C. Calegari, D.A. Cunha, M.J. Saad, L.A. Velloso, E.M. Rocha, Increased expression of advanced glycation end-products and their receptor, and activation of nuclear factor kappa-B in lacrimal glands of diabetic rats. Diabetologia 48, 2675–2681 (2005)
N. Ishida, G.N. Rao, M. del Cerro, J.V. Aquavella, Corneal nerve alterations in diabetes mellitus. Arch. Ophthalmol. 102, 1380–1384 (1984)
N.A. McNamara, R.J. Brand, K.A. Polse, W.M. Bourne, Corneal function during normal and high serum glucose levels in diabetes. Invest. Ophthalmol. Vis. Sci. 39, 3–17 (1998)
M. Wakuta, N. Morishige, T. Chikama, K. Seki, T. Nagano, T. Nishida, Delayed wound closure and phenotypic changes in corneal epithelium of the spontaneously diabetic Goto-Kakizaki rat. Invest. Ophthalmol. Vis. Sci. 48, 590–596 (2007)
V. Peponis, S. Bonovas, A. Kapranou et al., Conjunctival and tear film changes after vitamin C and E administration in non-insulin dependent diabetes mellitus. Med Sci Monit 10, CR213–CR217 (2004)
E.M. Rocha, M.H. de M. Lima, C.R. Carvalho, M.J. Saad, L.A. Velloso, Characterization of the insulin-signaling pathway in lacrimal and salivary glands of rats. Curr. Eye Res. 21, 833–842 (2000)
M. Goebbels, Tear secretion and tear film function in insulin dependent diabetics. Br. J. Ophthalmol. 84, 19–21 (2000)
M. Dogru, C. Katakami, M. Inoue, Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology 108, 586–592 (2001)
D.A. Cunha, E.M. Carneiro, C. Alves Mde et al., Insulin secretion by rat lacrimal glands: effects of systemic and local variables. Am. J. Physiol. Endocrinol. Metab. 289, E768–E775 (2005)
E.M. Rocha, D.A. Cunha, E.M. Carneiro, A.C. Boschero, M.J. Saad, L.A. Velloso, Identification of insulin in the tear film and insulin receptor and IGF-1 receptor on the human ocular surface. Invest. Ophthalmol. Vis. Sci. 43, 963–967 (2002)
M.G. Myers Jr., M.F. White, Insulin signal transduction and the IRS proteins. Annu. Rev. Pharmacol. Toxicol. 36, 615–658 (1996)
J.E. Pessin, A.R. Saltiel, Signaling pathways in insulin action: molecular targets of insulin resistance. J. Clin. Invest. 106, 165–169 (2000)
J.L. Evans, I.D. Goldfine, B.A. Maddux, G.M. Grodsky, Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr. Rev. 23, 599–622 (2002)
D.A. Cunha, M.C. de Alves, L.F. Stoppiglia et al., Extra-pancreatic insulin production in RAt lachrymal gland after streptozotocin-induced islet beta-cells destruction. Biochim. Biophys. Acta 1770, 1128–1135 (2007)
F.N. Nogueira, A.M. Carvalho, P.M. Yamaguti, J. Nicolau, Antioxidant parameters and lipid peroxidation in salivary glands of streptozotocin-induced diabetic rats. Clin. Chim. Acta 353, 133–139 (2005)
H. Daiyasu, H. Toh, Molecular evolution of the myeloperoxidase family. J. Mol. Evol. 51, 433–445 (2000)
B.B. Bromberg, M.M. Cripps, M.H. Welch, Peroxidase secretion by lacrimal glands from juvenile F344 rats. Invest. Ophthalmol. Vis. Sci. 30, 562–568 (1989)
P.K. De, Tissue distribution of constitutive and induced soluble peroxidase in rat. Purification and characterization from lacrimal gland. Eur. J. Biochem. 206, 59–67 (1992)
J.D. Rios, Y. Horikawa, L.L. Chen et al., Age-dependent alterations in mouse exorbital lacrimal gland structure, innervation and secretory response. Exp. Eye Res. 80, 477–491 (2005)
F. Passardi, G. Theiler, M. Zamocky et al., PeroxiBase: the peroxidase database. Phytochemistry 68, 1605–1611 (2007)
R. Brigelius-Flohe, Tissue-specific functions of individual glutathione peroxidases. Free Radic. Biol. Med. 27, 951–965 (1999)
A. Paliwal, P.K. De, Marked sexual dimorphism of lacrimal gland peroxidase in hamster: repression by androgens and estrogens. Biochem. Biophys. Res. Commun. 341, 1286–1293 (2006)
A. Paliwal, A.Q. Srikantan, P.K. Stephano, Androgen, estrogen and thyroid hormones repress tear peroxidase gene in lacrimal gland of hamster. Ocul Surf 3, S102 (2005)
A.G. Jorge, C.M. Modulo, A.C. Dias et al., Aspirin prevents diabetic oxidative changes in rat lacrimal gland structure and function. Endocrine 35, 189–197 (2009)
P. Zimmet, K.G. Alberti, J. Shaw, Global and societal implications of the diabetes epidemic. Nature 414, 782–787 (2001)
The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N. Engl. J. Med. 329, 977–986 (1993)
M. de C. Alves, J.B. Carvalheira, C.M. Modulo, E.M. Rocha, Tear film and ocular surface changes in diabetes mellitus. Arq. Bras. Oftalmol. 71, 96–103 (2008)
L.E. Hann, R.S. Kelleher, D.A. Sullivan, Influence of culture conditions on the androgen control of secretory component production by acinar cells from the rat lacrimal gland. Invest. Ophthalmol. Vis. Sci. 32, 2610–2621 (1991)
D. Barnes, G. Sato, Methods for growth of cultured cells in serum-free medium. Anal. Biochem. 102, 255–270 (1980)
X. Zhu, J. Wallman, Opposite effects of glucagon and insulin on compensation for spectacle lenses in chicks. Invest. Ophthalmol. Vis. Sci. 50, 24–36 (2009)
J. Nepp, C. Abela, I. Polzer, A. Derbolav, A. Wedrich, Is there a correlation between the severity of diabetic retinopathy and keratoconjunctivitis sicca? Cornea 19, 487–491 (2000)
M. Henricsson, A. Nilsson, L. Janzon, L. Groop, The effect of glycaemic control and the introduction of insulin therapy on retinopathy in non-insulin-dependent diabetes mellitus. Diabet. Med. 14, 123–131 (1997)
S. Saragas, R. Arffa, B. Rabin, J. Kronish, D. Miller, C. Mayman, Reversal of wound strength retardation by addition of insulin to corticosteroid therapy. Ann. Ophthalmol. 17, 428–430 (1985)
S.C. Leite, R.S. de Castro, M. Alves et al., Risk factors and characteristics of ocular complications, and efficacy of autologous serum tears after haematopoietic progenitor cell transplantation. Bone Marrow Transplant. 38, 223–227 (2006)
I.S. Zagon, M.S. Klocek, J.W. Sassani, P.J. McLaughlin, Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch. Ophthalmol. 125, 1082–1088 (2007)
D.A. Sullivan, L.E. Hann, Hormonal influence on the secretory immune system of the eye: endocrine impact on the lacrimal gland accumulation and secretion of IgA and IgG. J. Steroid Biochem. 34, 253–262 (1989)
E. Adeghate, A. Ponery, R. Hameed, Notes on the effect of diabetes mellitus on the morphology and function of the rat lacrimal gland. Ocul Surf 3, S42 (2005)
D.A. Sullivan, L.E. Hann, L. Yee, M.R. Allansmith, Age- and gender-related influence on the lacrimal gland and tears. Acta Ophthalmol. (Copenh.) 68, 188–194 (1990)
E. Guerriero, J. Chen, Y. Sado et al., Loss of alpha3(IV) collagen expression associated with corneal keratocyte activation. Invest. Ophthalmol. Vis. Sci. 48, 627–635 (2007)
T. Kondo, D. Vicent, K. Suzuma et al., Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. J. Clin. Invest. 111, 1835–1842 (2003)
M.S. Al Shamsi, A. Amin, E. Adeghate, Beneficial effect of vitamin E on the metabolic parameters of diabetic rats. Mol. Cell. Biochem. 261, 35–42 (2004)
L. Zheng, S.J. Howell, D.A. Hatala, K. Huang, T.S. Kern, Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes 56, 337–345 (2007)
P.I. Moreira, M.S. Santos, C. Sena, R. Seica, C.R. Oliveira, Insulin protects against amyloid beta-peptide toxicity in brain mitochondria of diabetic rats. Neurobiol. Dis. 18, 628–637 (2005)
W. Sun, C. Gerhardinger, Z. Dagher, T. Hoehn, M. Lorenzi, Aspirin at low-intermediate concentrations protects retinal vessels in experimental diabetic retinopathy through non-platelet-mediated effects. Diabetes 54, 3418–3426 (2005)
S. Mahay, E. Adeghate, M.Z. Lindley, C.E. Rolph, J. Singh, Streptozotocin-induced type 1 diabetes mellitus alters the morphology, secretory function and acyl lipid contents in the isolated rat parotid salivary gland. Mol. Cell. Biochem. 261, 175–181 (2004)
C. Ding, M. MacVeigh, M. Pidgeon et al., Unique ultrastructure of exorbital lacrimal glands in male NOD and BALB/c mice. Curr. Eye Res. 31, 13–22 (2006)
V. Peponis, M. Papathanasiou, A. Kapranou et al., Protective role of oral antioxidant supplementation in ocular surface of diabetic patients. Br. J. Ophthalmol. 86, 1369–1373 (2002)
M.G. Bonini, A.G. Siraki, S. Bhattacharjee, R.P. Mason, Glutathione-induced radical formation on lactoperoxidase does not correlate with the enzyme’s peroxidase activity. Free Radic. Biol. Med. 42, 985–992 (2007)
Q. Guo, C.D. Detweiler, R.P. Mason, Protein radical formation during lactoperoxidase-mediated oxidation of the suicide substrate glutathione: immunochemical detection of a lactoperoxidase radical-derived 5, 5-dimethyl-1-pyrroline N-oxide nitrone adduct. J. Biol. Chem. 279, 13272–13283 (2004)
A.C. Dias, C.M. Modulo, A.G. Jorge et al., Influence of thyroid hormone on thyroid hormone receptor beta-1 expression and lacrimal gland and ocular surface morphology. Invest. Ophthalmol. Vis. Sci. 48, 3038–3042 (2007)
M.V. de Rojas, M.T. Rodriguez, J.A. Ces Blanco, M.S. Salorio, Impression cytology in patients with keratoconjunctivitis sicca. Cytopathology 4, 347–355 (1993)
J.N.M.V. Barros, J.A. Pereira Gomes, D. Freitas, A.L.H. Lima, Impression cytology of the ocular surface: examination technique and staining procedure. Arq. Bras. Oftalmol. 64, 127–131 (2001)
H. Esterbauer, K.H. Cheeseman, Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 186, 407–421 (1990)
D. Zoukhri, C.L. Kublin, Impaired neurotransmitter release from lacrimal and salivary gland nerves of a murine model of Sjogren’s syndrome. Invest. Ophthalmol. Vis. Sci. 42, 925–932 (2001)
Acknowledgments
The authors thank Mrs. Vani Maria Alves Correa for technical assistance with this work and Mrs. Electra Green for reviewing the manuscript. This work was financially supported by CAPES, CNPq, and FAPESP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Módulo, C.M., Jorge, A.G., Dias, A.C. et al. Influence of insulin treatment on the lacrimal gland and ocular surface of diabetic rats. Endocr 36, 161–168 (2009). https://doi.org/10.1007/s12020-009-9208-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-009-9208-9