Abstract
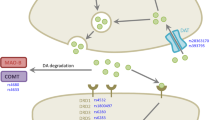
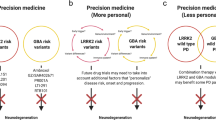
Parkinson’s disease (PD) is a complex neurodegenerative disorder characterized by a progressive loss of dopamine neurons of the central nervous system. The disease determines a significant disability due to a combination of motor symptoms such as bradykinesia, rigidity and rest tremor and non-motor symptoms such as sleep disorders, hallucinations, psychosis and compulsive behaviors. The current therapies consist in combination of drugs acting to control only the symptoms of the illness by the replacement of the dopamine lost. Although patients generally receive benefits from this symptomatic pharmacological management, they also show great variability in drug response in terms of both efficacy and adverse effects. Pharmacogenetic studies highlighted that genetic factors play a relevant influence in this drug response variability. In this review, we tried to give an overview of the recent progresses in the pharmacogenetics of PD, reporting the major genetic factors identified as involved in the response to drugs and highlighting the potential use of some of these genomic variants in the clinical practice. Many genes have been investigated and several associations have been reported especially with adverse drug reactions. However, only polymorphisms in few genes, including DRD2, COMT and SLC6A3, have been confirmed as associated in different populations and in large cohorts. The identification of genomic biomarkers involved in drug response variability represents an important step in PD treatment, opening the prospective of more personalized therapies in order to identify, for each person, the better therapy in terms of efficacy and toxicity and to improve the PD patients’ quality of life.

Similar content being viewed by others
References
Acuña, G., Foernzler, D., Leong, D., Rabbia, M., Smit, R., Dorflinger, E., et al. (2002). Pharmacogenetic analysis of adverse drug effect reveals genetic variant for susceptibility to liver toxicity. The Pharmacogenomics Journal, 2(5), 327–334.
Agúndez, J. A., García-Martín, E., Alonso-Navarro, H., & Jiménez-Jiménez, F. J. (2013). Anti-Parkinson’s disease drugs and pharmacogenetic considerations. Expert Opinion on Drug Metabolism & Toxicology, 9(7), 859–874.
Ahlskog, J. E., & Muenter, M. D. (2001). Frequency of levodopa related dyskinesias and motor fluctuations as estimated from the cumulative literature. Movement Disorders Journal, 16(3), 448–458.
Alonso-Navarro, H., Jimenez-Jimenez, F. J., Garcia-Martin, E., & Agundez, J. A. (2014). Genomic and pharmacogenomic biomarkers of Parkinson’s disease. Current Drug Metabolism, 15(2), 129–181.
Altmann, V., Schumacher-Schuh, A. F., Rieck, M., Callegari-Jacques, S. M., Rieder, C. R., & Hutz, M. H. (2016). Influence of genetic, biological and pharmacological factors on levodopa dose in Parkinson’s disease. Pharmacogenomics, 17(5), 481–488.
Arbouw, M. E., Movig, K. L., Egberts, T. C., Poels, P. J., van Vugt, J. P., Wessels, J. A., et al. (2009). Clinical and pharmacogenetic determinants for the discontinuation of non-ergoline dopamine agonists in Parkinson’s disease. European Journal of Clinical Pharmacology, 65(12), 1245–1251.
Arbouw, M. E., Movig, K. L., Guchelaar, H. J., Poels, P. J., van Vugt, J. P., Neef, C., et al. (2008). Discontinuation of ropinirole and pramipexole in patients with Parkinson’s disease: Clinical practice versus clinical trials. European Journal of Clinical Pharmacology, 64(10), 1021–1026.
Baik, J. H. (2013). Dopamine signaling in food addiction: Role of dopamine D2 receptors. BMB Reports, 46(11), 519–526.
Becker, M. L., Visser, L. E., van Schaik, R. H., Hofman, A., Uitterlinden, A. G., & Stricker, B. H. (2011). OCT1 polymorphism is associated with response and survival time in anti-Parkinsonian drug users. Neurogenetics, 12(1), 79–82.
Beinfeld, M. C. (2001). An introduction to neuronal cholecystokinin. Peptides, 22(8), 1197–1200.
Berry, M. D., Juorio, A. V., Li, X. M., & Boulton, A. A. (1996). Aromatic l-amino acid decarboxylase: A neglected and misunderstood enzyme. Neurochemical Research, 21(9), 1075–1087.
Besch, R., Giovannangeli, C., & Degitz, K. (2004). Triplex-forming oligonucleotides—Sequence-specific DNA ligands as tools for gene inhibition and for modulation of DNA-associated functions. Current Drug Targets, 5(8), 691–703.
Bezard, E., Brotchie, J. M., & Gross, C. E. (2001). Pathophysiology of levodopa induced dyskinesia: Potential for new therapies. Nature Reviews Neuroscience, 2(8), 577–588.
Białecka, M., Droździk, M., Kłodowska-Duda, G., Honczarenko, K., Gawrońska-Szklarz, B., Opala, G., et al. (2004). The effect of monoamine oxidase B (MAOB) and catechol-Omethyltransferase (COMT) polymorphisms on levodopa therapy in patients with sporadic Parkinson’s disease. Acta Neurologica Scandinavica, 110(4), 260–266.
Bialecka, M., Klodowska-Duda, G., Honczarenko, K., Gawrońska-Szklarz, B., Opala, G., Safranow, K., et al. (2007). Polymorphisms of catechol-O-methyltransferase (COMT), monoamine oxidase B (MAOB), N-acetyltransferase 2 (NAT2) and cytochrome P450 2D6 (CYP2D6) gene in patients with early onset of Parkinson’s disease. Parkinsonism & Related Disorders, 13(4), 224–229.
Bialecka, M., Kurzawski, M., Klodowska-Duda, G., Opala, G., Tan, E. K., & Drozdzik, M. (2008). The association of functional catechol-O-methyltransferase haplotypes with risk of Parkinson’s disease, levodopa treatment response, and complications. Pharmacogenetics and Genomics, 18(9), 815–821.
Bond, C., LaForge, K. S., Tian, M., Melia, D., Zhang, S., Borg, L., et al. (1998). Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: Possible implications for opiate addiction. Proceedings of the National Academy of Sciences USA, 95(16), 9608–9613.
Borges, N. (2005). Tolcapone in Parkinson’s disease: Liver toxicity and clinical efficacy. Expert Opinion in Drug Safety, 4(1), 69–73.
Børglum, A. D., Kirov, G., Craddock, N., Mors, O., Muir, W., Murray, V., et al. (2003). Possible parent-of-origin effect of Dopa decarboxylase in susceptibility to bipolar affective disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 117B(1), 18–22.
Brotchie, J. M., Lee, J., & Venderova, K. (2005). Levodopa-induced dyskinesia in Parkinson’s disease. Journal of Neural Transmission, 112(3), 359–391.
Camicioli, R., Rajput, A., Rajput, M., Reece, C., Payami, H., & Hao, C. (2005). Apolipoprotein E epsilon4 and catechol-O-methyltransferase alleles in autopsyproven Parkinson’s disease: Relationship to dementia and hallucinations. Movement Disorders, 20(8), 989–994.
Cargnin, S., Jommi, C., Canonico, P. L., Genazzani, A. A., & Terrazzino, S. (2014). Diagnostic accuracy of HLA-B*57:01 screening for the prediction of abacavir hypersensitivity and clinical utility of the test: A meta-analytic review. Pharmacogenomics, 15(7), 963–976.
Chapman, J., Korczyn, A. D., Karussis, D. M., & Michaelson, D. M. (2001). The effects of APOE genotype on age at onset and progression of neurodegenerative diseases. Neurology, 57(8), 1482–1485.
Chaudhuri, R., & Schapira, A. (2009). Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. The Lancet Neurology, 8(5), 464–474.
Cheshire, P., Bertram, K., Ling, H., O’Sullivan, S. S., Halliday, G., McLean, C., et al. (2014). Influence of single nucleotide polymorphisms in COMT, MAO-A and BDNF genes on dyskinesias and levodopa use in Parkinson’s disease. Neurodegenerative Disease, 13(1), 24–28.
Clarke, C. E., & Guttman, M. (2002). Dopamine agonist monotherapy in Parkinson’s disease. Lancet, 360(9347), 1767–1769.
Conde, L., Vaquerizas, J. M., Dopazo, H., Arbiza, L., Reumers, J., Rousseau, F., et al. (2006). PupaSuite: Finding functional single nucleotide polymorphisms for large-scale genotyping purposes. Nucleic Acids Research, 34(Web Server issue), W621-5.
Contin, M., Martinelli, P., Mochi, M., Riva, R., Albani, F., & Baruzzi, A. (2005). Genetic polymorphism of catechol-O-methyltransferase and levodopa pharmacokinetic–pharmacodynamic pattern in patients with Parkinson’s disease. Movement Disorders, 20(6), 734–739.
Corvol, J. C., Bonnet, C., Charbonnier-Beaupel, F., Bonnet, A. M., Fiévet, M. H., Bellanger, A., et al. (2011). The COMT Val158Met polymorphism affects the response to entacapone in Parkinson’s disease: A randomized crossover clinical trial. Annals of Neurology, 69(1), 111–118.
Cummings, J. L. (1991). Behavioral complications of drug treatment of Parkinson’s disease. Journal of the American Geriatrics Society, 39(7), 708–716.
Dardou, D., Dassesse, D., Cuvelier, L., Deprez, T., De Ryck, M., & Schiffmann, S. N. (2011). Distribution of SV2C mRNA and protein expression in the mouse brain with a particular emphasis on the basal ganglia system. Brain Research, 1367, 130–145.
De Lau, L., & Breteler, M. (2006). Epidemiology of Parkinson’s disease. The Lancet Neurology, 5(6), 525–535.
De Lau, L. M., Verbaan, D., Marinus, J., Heutink, P., & van Hilten, J. J. (2012). Catechol-O-methyltransferase Val158Met and the risk of dyskinesias in Parkinson’s disease. Movement Disorders, 27(1), 132–135.
De Luca, V., Annesi, G., De Marco, E. V., de Bartolomeis, A., Nicoletti, G., Pugliese, P., et al. (2009). HOMER1 promoter analysis in Parkinson’s disease: Association study with psychotic symptoms. Neuropsychobiology, 59(4), 239–245.
Devos, D., Lejeune, S., Cormier-Dequaire, F., Tahiri, K., Charbonnier-Beaupel, F., Rouaix, N., et al. (2014). Dopa-decarboxylase gene polymorphisms affect the motor response to L-dopa in Parkinson’s disease. Parkinsonism & Related Disorders, 20(2), 170–175.
Džoljić, E., Novaković, I., Krajinovic, M., Grbatinić, I., & Kostić, V. (2015). Pharmacogenetics of drug response in Parkinson’s disease. The International Journal of Neuroscience, 125(9), 635–644.
Egan, M. F., Kojima, M., Callicott, J. H., Goldberg, T. E., Kolachana, B. S., Bertolino, A., et al. (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell, 112(2), 257–269.
Fahn, S., Oakes, D., Shoulson, I., Kieburtz, K., Rudolph, A., Lang, A., et al. (2004). Levodopa and the progression of Parkinson’s disease. The New England Journal of Medicine, 351(24), 2498–2508.
Feldman, B., Chapman, J., & Korczyn, A. D. (2006). Apolipoprotein epsilon4 advances appearance of psychosis in patients with Parkinson’s disease. Acta Neurologica Scandinavica, 113(1), 14–17.
Fénelon, G., & Alves, G. (2010). Epidemiology of psychosis in Parkinson’s disease. Journal of the Neurological Sciences, 289(1–2), 12–17.
Ferrari, M., Martignoni, E., Blandini, F., Riboldazzi, G., Bono, G., Marino, F., et al. (2012). Association of UDP-glucuronosyltransferase 1A9 polymorphisms with adverse reactions to catechol-O-methyltransferase inhibitors in Parkinson’s disease patients. European Journal of Clinical Pharmacology, 68(11), 1493–1499.
Fisher, A., Croft-Baker, J., Davis, M., Purcell, P., & McLean, A. J. (2002). Entacapone-induced hepatotoxicity and hepatic dysfunction. Movement Disorders, 17(6), 1362–1365.
Foltynie, T., Cheeran, B., Williams-Gray, C. H., Edwards, M. J., Schneider, S. A., Weinberger, D., et al. (2009). BDNF val66met influences time to onset of levodopa induced dyskinesia in Parkinson’s disease. Journal of Neurology, Neurosurgery and Psychiatry, 80(2), 141–144.
Fox, S. H., Katzenschlager, R., Lim, S. Y., Ravina, B., Seppi, K., Coelho, M., et al. (2011). The movement disorder society evidence-based medicine review update: Treatments for the motor symptoms of Parkinson’s disease. Movement Disorders, 26(Suppl 3), S2–S41.
Fox, S. H., & Lang, A. E. (2008). Levodopa-related motor complications—Phenomenology. Movement Disorders, 23(Suppl. 3), S509–S514.
Frauscher, B., Högl, B., Maret, S., Wolf, E., Brandauer, E., Wenning, G. K., et al. (2004). Association of daytime sleepiness with COMT polymorphism in patients with Parkinson disease: A pilot study. Sleep, 27(4), 733–736.
Fujii, C., Harada, S., Ohkoshi, N., Hayashi, A., Yoshizawa, K., Ishizuka, C., et al. (1999). Association between polymorphism of the cholecystokinin gene and idiopathic Parkinson’s disease. Clinical Genetics, 56(5), 394–399.
Garcia-Borreguero, D., Schwarz, C., Larrosa, O., de la Llave, Y., & Garcia de Yébenes, J. (2003). L-DOPA-induced excessive daytime sleepiness in PD: A placebo-controlled case with MSLT assessment. Neurology, 61(7), 1008–1010.
Garcia-Ruiz, P. J., Martinez Castrillo, J. C., Alonso-Canovas, A., Herranz Barcenas, A., Vela, L., Sanchez Alonso, P., et al. (2014). Impulse control disorder in patients with Parkinson’s disease under dopamine agonist therapy: A multicentre study. Journal of Neurology, Neurosurgery and Psychiatry, 85(8), 840–844.
Goetz, C. G., Burke, P. F., Leurgans, S., Berry-Kravis, E., Blasucci, L. M., Raman, R., et al. (2001). Genetic variation analysis in Parkinson disease patients with and without hallucinations: Case–control study. Archives of Neurology, 58(2), 209–213.
Goldman, J. G., Goetz, C. G., Berry-Kravis, E., Leurgans, S., & Zhou, L. (2004). Genetic polymorphisms in Parkinson disease subjects with and without hallucinations: An analysis of the cholecystokinin system. Archives Neurology, 61(8), 1280–1284.
Goldman, J. G., Marr, D., Zhou, L., Ouyang, B., Leurgans, S. E., Berry-Kravis, E., et al. (2011). Racial differences may influence the role of cholecystokinin polymorphisms in Parkinson’s disease hallucinations. Movement Disorders, 26(9), 1781–1782.
Goudreau, J. L., Maraganore, D. M., Farrer, M. J., Lesnick, T. G., Singleton, A. B., Bower, J. H., et al. (2002). Case-control study of dopamine transporter-1, monoamine oxidase-B, and catechol-O-methyl transferase polymorphisms in Parkinson’s disease. Movement Disorders, 17(6), 1305–1311.
Guay, D. R. (2006). Rasagiline (TVP-1012): A new selective monoamine oxidase inhibitor for Parkinson’s disease. The American Journal of Geriatric Pharmacotherapy, 4, 330–346.
Guerini, F. R., Beghi, E., Riboldazzi, G., Zangaglia, R., Pianezzola, C., Bono, G., et al. (2009). BDNF Val66Met polymorphism is associated with cognitive impairment in Italian patients with Parkinson’s disease. European Journal of Neurology, 16(11), 1240–1245.
Guntaka, R. V., Varma, B. R., & Weber, K. T. (2003). Triplex-forming oligonucleotides as modulators of gene expression. The International Journal of Biochemistry & Cell Biology, 35(1), 22–31.
Hardoff, R., Sula, M., Tamir, A., Soil, A., Front, A., Badarna, S., et al. (2001). Gastric emptying time and gastric motility in patients with Parkinson’s disease. Movement Disorder, 16(6), 1041–1047.
Harhangi, B. S., de Rijk, N. C., Van Duijn, C. M., Van Broeckhoven, C., Hofman, A., & Breteler, M. M. B. (2000). APOE and the risk of PD with or without dementia in a population based study. Neurology, 54(6), 1272–1276.
Hill-Burns, E. M., Singh, N., Ganguly, P., Hamza, T. H., Montimurro, J., Kay, D. M., et al. (2013). A genetic basis for the variable effect of smoking/nicotine on Parkinson’s disease. Pharmacogenomics Journal, 13(6), 530–537.
Högl, B., Seppi, K., Brandauer, E., Glatzlm, S., Frauscher, B., Niedermüller, U., et al. (2003). Increased daytime sleepiness in Parkinson’s disease: A questionnaire survey. Movement Disorders, 8(3), 319–323.
Hungs, M., & Mignot, E. (2001). Hypocretin/orexin, sleep and narcolepsy. BioEssays, 23(5), 397–408.
Ivanova, S. A., Loonen, A. J., Pechlivanoglou, P., Freidin, M. B., Al Hadithy, A. F., Rudikov, E. V., et al. (2012). NMDA receptor genotypes associated with the vulnerability to develop dyskinesia. Translational. Psychiatry, 2, e67.
Jankovic, J. (2008). Parkinson’s disease: Clinical features and diagnosis. Journal of Neurology, Neurosurgery and Psychiatry, 79(4), 368–376.
Jeanneteau, F., Funalot, B., Jankovic, J., Deng, H., Lagarde, J. P., Lucotte, G., et al. (2006). A functional variant of the dopamine D (3) receptor is associated with risk and age-at-onset of essential tremor. Proceedings of the National Academy of Sciences USA, 103(28), 10753–10758.
Jiménez-Jiménez, F. J., Alonso-Navarro, H., García-Martín, E., & Agúndez, J. A. (2016). Advances in understanding genomic markers and pharmacogenetics of Parkinson’s disease. Expert Opinion on Drug Metabolism & Toxicology, 12(4), 433–448.
Kaiser, R., Hofer, A., Grapengiesser, A., Gasser, T., Kupsch, A., Roots, I., et al. (2003). L-dopa-induced adverse effects in PD and dopamine transporter gene polymorphism. Neurology, 60(11), 1750–1755.
Kalinderi, K., Fidani, L., Katsarou, Z., & Bostantjopoulou, S. (2011). Pharmacological treatment and the prospect of pharmacogenetics in Parkinson’s disease. International Journal of Clinical Practice, 65(12), 1289–1294.
Kaplan, N., Vituri, A., Korczyn, A. D., Cohen, O. S., Inzelberg, R., Yahalom, G., et al. (2014). Sequence variants in SLC6A3, DRD2, and BDNF genes and time to levodopa-induced dyskinesias in Parkinson’s disease. Journal of Molecular Neuroscience, 53(2), 183–188.
Kaplowitz, N. (2005). Idiosyncratic drug hepatotoxicity. Nature Reviews Drug Discovery, 4(6), 489–499.
Kempster, P. A., O’Sullivan, S. S., Holton, J. L., Revesz, T., & Lees, A. J. (2010). Relationships between age and late progression of Parkinson’s disease: A clinico-pathological study. Brain, 133(Pt 6), 1755–1762.
Kilduff, T. S., & Peyron, C. (2000). The hypocretin/orexin ligand-receptor system: Implications for sleep and sleep disorders. Trends in Neurosciences, 23(8), 359–365.
Koepsell, H., Lips, K., & Volk, C. (2007). Polyspecific organic cation transporters: Structure, function, physiological roles, and biopharmaceutical implications. Pharmaceutical Research, 24(7), 1227–1251.
Krishnamoorthy, S., Rajan, R., Banerjee, M., Kumar, H., Sarma, G., Krishnan, S., et al. (2016). Dopamine D3 receptor Ser9Gly variant is associated with impulse control disorders in Parkinson’s disease patients. Parkinsonism & Related Disorders, 30, 13–17.
Kurzawski, M., Białecka, M., & Droździk, M. (2015). Pharmacogenetic considerations in the treatment of Parkinson’s disease. Neurodegenerative Disease Management, 5(1), 27–35.
Labandeira-Garcia, J. L., Rodriguez-Pallares, J., Dominguez- Meijide, A., Valenzuela, R., Villar-Cheda, B., et al. (2013). Dopamine-angiotensin interactions in the basal ganglia and their relevance for Parkinson’s disease. Movement Disorders, 28(10), 1337–1342.
Lee, J. Y., Cho, J., Lee, E. K., Park, S. S., & Jeon, B. S. (2011). Differential genetic susceptibility in diphasic and peak-dose dyskinesias in Parkinson’s disease. Movement Disorders, 26(1), 73–79.
Lee, M. S., Lyoo, C. H., Ulmanen, I., Syvänen, A. C., & Rinne, J. O. (2001). Genotypes of catechol-O-methyltransferase and response to levodopa treatment in patients with Parkinson’s disease. Neuroscience Letters, 298(2), 131–134.
Li, Y. J., Scott, W. K., Hedges, D. J., Zhang, F., Gaskell, P. C., Nance, M. A., et al. (2002). Age at onset in two common neurodegenerative diseases is genetically controlled. American Journal of Human Genetics, 70(4), 985–993.
Lin, J. J., Yueh, K. C., Lin, S. Z., Harn, H. J., & Liu, J. T. (2007). Genetic polymorphism of the angiotensin converting enzyme and L-dopa-induced adverse effects in Parkinson’s disease. Journal of the Neurological Science, 252(2), 130–134.
Linazasoro, G. (2005). New ideas on the origin of L-dopa-induced dyskinesias: Age, genes and neural plasticity. Trends in Pharmacological Science, 26(8), 391–397.
Liu, Y. Z., Tang, B. S., Yan, X. X., Liu, J., Ouyang, D. S., Nie, L. N., et al. (2009). Association of the DRD2 and DRD3 polymorphisms with response to pramipexole in Parkinson’s disease patients. European Journal of Clinical Pharmacology, 65(7), 679–683.
Luo, P., Li, X., Fei, Z., & Poon, W. (2012). Scaffold protein Homer 1: Implications for neurological diseases. Neurochemistry International, 61(5), 731–738.
Makoff, A. J., Graham, J. M., Arranz, M. J., Forsyth, J., Li, T., Aitchison, K. J., et al. (2000). Association study of dopamine receptor gene polymorphisms with drug-induced hallucinations in patients with idiopathic Parkinson’s disease. Pharmacogenetics, 10(1), 43–48.
Martignoni, E., Cosentino, M., Ferrari, M., Porta, G., Mattarucchi, E., Marino, F., et al. (2005). Two patients with COMT inhibitor-induced hepatic dysfunction and UGT1A9 genetic polymorphism. Neurology, 65(11), 1820–1822.
Masellis, M., Collinson, S., Freeman, N., Tampakeras, M., Levy, J., Tchelet, A., et al. (2016). Dopamine D2 receptor gene variants and response to rasagiline in early Parkinson’s disease: A pharmacogenetic study. Brain, 139(Pt 7), 2050–2062.
Mhyre, T. R., Boyd, J. T., Hamill, R. W., & Maguire-Zeiss, K. A. (2012). Parkinson’s disease. SubCellular Biochemistry, 65, 389–455.
Mishina, M., Ishiwata, K., Naganawa, M., Kimura, Y., Kitamura, S., & Suzuki, M. (2011). Adenosine A(2A) receptors measured with [C]TMSX PET in the striata of Parkinson’s disease patients. PLoS ONE, 6(2), e17338.
Momose, Y., Murata, M., Kobayashi, K., Tachikawa, M., Nakabayashi, Y., & Kanazawa, I. (2002). Association studies of multiple candidate genes for Parkinson’s disease using single nucleotide polymorphisms. Annals of Neurology, 51(1), 133–136.
Moore, T. J., Glenmullen, J., & Mattison, D. R. (2014). Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA Internal Medicine, 174(12), 1930–1933.
Moreau, C., Meguig, S., Corvol, J. C., Labreuche, J., Vasseur, F., Duhamel, A., et al. (2015). Polymorphism of the dopamine transporter type 1 gene modifies the treatment response in Parkinson’s disease. Brain, 138(Pt 5), 1271–1283.
Morgante, F., Espay, A. J., Gunraj, C., Lang, A. E., & Chen, R. (2006). Motor cortex plasticity in Parkinson’s disease and levodopa-induced dyskinesias. Brain, 129(Pt 4), 1059–1069.
Murer, M. G., Yan, Q., & Raisman-Vozari, R. (2001). Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Progress in Neurobiology, 63(1), 71–124.
Obeso, J. A., Rodriguez-Oroz, M. C., Goetz, C. G., Marin, C., Kordower, J. H., Rodriguez, M., et al. (2010). Missing pieces in the Parkinson’s disease puzzle. Nature Medicine, 16(6), 653–661.
Olanow, C. W. (2000). Tolcapone and hepatotoxic effects. Tasmar Advisory Panel. Archives of Neurology, 57(2), 263–267.
Olanow, C., Stern, M., & Sethi, K. (2009). The scientific and clinical basis for the treatment of Parkinson disease. Neurology, 72(Suppl 4), S1–S136.
Oliveri, R. L., Annesi, G., Zappia, M., Civitelli, D., Montesanti, R., Branca, D., et al. (1999). Dopamine D2 receptor gene polymorphism and the risk of levodopa-induced dyskinesias in PD. Neurology, 53(7), 1425–1430.
Overeem, S., van Hilten, J. J., Ripley, B., Mignot, E., Nishino, S., & Lammers, G. J. (2002). Normal hypocretin-1 levels in Parkinson’s disease patients with excessive daytime sleepiness. Neurology, 58(3), 498–499.
Pascale, E., Purcaro, C., Passarelli, E., Guglielmi, R., Vestri, A. R., Passarelli, F., et al. (2009). Genetic polymorphism of angiotensin-converting enzyme is not associated with the development of Parkinson’s disease and of l-dopa-induced adverse effects. Journal of the Neurological Sciences, 276(1–2), 18–21.
Paus, S., Gadow, F., Knapp, M., Klein, C., Klockgether, T., & Wüllner, U. (2009). Motor complications in patients form the German Competence Network on Parkinson’s disease and the DRD3 Ser9Gly polymorphism. Movement Disorders, 24(7), 1080–1084.
Paus, S., Grünewald, A., Klein, C., Knapp, M., Zimprich, A., Janetzky, B., et al. (2008). The DRD2 TaqIA polymorphism and demand of dopaminergic medication in Parkinson’s disease. Movement Disorders, 23(4), 599–602.
Paus, S., Seeger, G., Brecht, H. M., Köster, J., El-Faddagh, M., Nöthen, M. M., et al. (2004). Association study of dopamine D2, D3, D4 receptor and serotonin transporter gene polymorphisms with sleep attacks in Parkinson’s disease. Movement Disorders, 19(6), 705–707.
Payami, H., & Factor, S. A. (2014). Promise of pharmacogenomics for drug discovery, treatment and prevention of Parkinson’s disease. A perspective. Neurotherapeutics, 11(1), 111–116.
Picconi, B., Paillé, V., Ghiglieri, V., Bagetta, V., Barone, I., Lindgren, H. S., et al. (2008). L-DOPA dosage is critically involved in dyskinesia via loss of synaptic depotentiation. Neurobiology of Disease, 29(2), 327–335.
Ramlackhansingh, A. F., Bose, S. K., Ahmed, I., Turkheimer, F. E., Pavese, N., & Brooks, D. J. (2011). Adenosine 2A receptor availability in dyskinetic and nondyskinetic patients with Parkinson disease. Neurology, 76(21), 1811–1816.
Rieck, M., Schumacher-Schuh, A. F., Altmann, V., Callegari-Jacques, S. M., Rieder, C. R., & Hutz, M. H. (2016). Association between DRD2 and DRD3 gene polymorphisms and gastrointestinal symptoms induced by levodopa therapy in Parkinson’s disease. The Pharmacogenomics Journal. https://doi.org/10.1038/tpj.2016.79.
Rieck, M., Schumacher-Schuh, A. F., Altmann, V., Francisconi, C. L., Fagundes, P. T., Monte, T. L., et al. (2012). DRD2 haplotype is associated with dyskinesia induced by levodopa therapy in Parkinson’s disease patients. Pharmacogenomics, 13(15), 1701–1710.
Rieck, M., Schumacher-Schuh, A. F., Callegari-Jacques, S. M., Altmann, V., Schneider Medeiros, M., Rieder, C. R., et al. (2015). Is there a role for ADORA2A polymorphisms in levodopa-induced dyskinesia in Parkinson’s disease patients? Pharmacogenomics, 16(6), 573–582.
Rissling, I., Geller, F., Bandmann, O., Stiasny-Kolster, K., Körner, Y., Meindorfner, C., et al. (2004). Dopamine receptor gene polymorphisms in Parkinson’s disease patients reporting “sleep attacks”. Movement Disorders, 19(11), 1279–1284.
Rissling, I., Korner, Y., Geller, F., Stiasny-Kolster, K., Oertel, W. H., & Moller, J. C. (2005). Preprohypocretin polymorphisms in Parkinson disease patients reporting “sleep attacks”. Sleep, 28(7), 871–875.
Rotzinger, S., Bush, D. E., & Vaccarino, F. J. (2002). Cholecystokinin modulation of mesolimbic dopamine function: Regulation of motivated behaviour. Pharmacology and Toxicology, 91(6), 404–413.
Rye, D. B., & Jankovic, J. (2002). Emerging views of dopamine in modulating sleep/wake state from an unlikely source: PD. Neurology, 58(3), 341–346.
Schiffmann, S. N., Fisone, G., Moresco, R., Cunha, R. A., & Ferre, S. (2007). Adenosine A2A receptors and basal ganglia physiology. Progress in Neurobiology, 83(5), 277–292.
Schumacher-Schuh, A. F., Altmann, V., Rieck, M., Tovo-Rodrigues, L., Monte, T. L., Callegari-Jacques, S. M., et al. (2014a). Association of common genetic variants of HOMER1 gene with levodopa adverse effects in Parkinson’s disease patients. The Pharmacogenomics Journal, 14(3), 289–294.
Schumacher-Schuh, A. F., Francisconi, C., Altmann, V., Monte, T. L., Callegari-Jacques, S. M., Rieder, C. R., et al. (2013). Polymorphisms in the dopamine transporter gene are associated with visual hallucinations and levodopa equivalent dose in Brazilians with Parkinson’s disease. Internal Journal of Neuropsychopharmacology, 16(6), 1251–1258.
Schumacher-Schuh, A. F., Rieder, C. R., & Hutz, M. H. (2014b). Parkinson’s disease pharmacogenomics: New findings and perspectives. Pharmacogenomics, 15(9), 1253–1271.
Stavitsky, K., & Cronin-Golomb, A. (2011). Sleep quality in Parkinson disease: An examination of clinical variables. Cognitive and Behavioral Neurology, 24(2), 43–49.
Strong, J. A., Dalvi, A., Revilla, F. J., Sahay, A., Samaha, F. J., Welge, J. A., et al. (2006). Genotype and smoking history affect risk of levodopa-induced dyskinesias in Parkinson’s Disease. Movement Disorders, 21(5), 654–659.
Studler, J. M., Javoy-Agid, F., Cesselin, F., Legrand, J. C., & Agid, Y. (1982). CCK-8- Immunoreactivity distribution in human brain: Selective decrease in the substantia nigra from parkinsonian patients. Brain Research, 243(1), 176–179.
Tao-Cheng, J. H. (2007). Ultrastructural localization of active zone and synaptic vesicle proteins in a preassembled multi-vesicle transport aggregate. Neuroscience, 150, 575–584.
Thanvi, B., Lo, N., & Robinson, T. (2007). Levodopa-induced dyskinesia in parkinson’s disease: Clinical features, pathogenesis, prevention and treatment. Postgraduate Medical Journal, 83(980), 384–388.
Thomas, U. (2002). Modulation of synaptic signalling complexes by Homer proteins. Journal of Neurochemistry, 81(3), 407–413.
Vallelunga, A., Flaibani, R., Formento-Dojot, P., Biundo, R., Facchini, S., & Antonini, A. (2012). Role of genetic polymorphisms of the dopaminergic system in Parkinson’s disease patients with impulse control disorders. Parkinsonism & Related Disorders, 18(4), 397–399.
Van de Giessen, E., de Win, M. M., Tanck, M. W., van den Brink, W., Baas, F., & Booij, J. (2009). Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. Journal of Nuclear Medicine, 50(1), 45–52.
Villeneuve, L., Girard, H., Fortier, L. C., Gagné, J. F., & Guillemette, C. (2003). Novel functional polymorphisms in the UGT1A7 and UGT1A9 glucuronidating enzymes in Caucasian and African-American subjects and their impact on the metabolism of 7-ethyl-10-hydroxycamptothecin and flavopiridol anticancer drugs. The Journal of Pharmacology and Experimental Therapeutics, 307(1), 117–128.
Wang, J., Liu, Z. L., & Chen, B. (2001). Association study of dopamine D2, D3 receptor gene polymorphisms with motor fluctuations in PD. Neurology, 56(12), 1757–1759.
Wang, J., Si, Y. M., Liu, Z. L., & Yu, L. (2003). Cholecystokinin, cholecystokinin-A receptor and cholecystokinin-B receptor gene polymorphisms in Parkinson’s disease. Pharmacogenetics, 13(6), 365–369.
Watanabe, M., Harada, S., Nakamura, T., Ohkoshi, N., Yoshizawa, K., Hayashi, A., et al. (2003). Association between catechol-O-methyltransferase gene polymorphisms and wearing-off and dyskinesia in Parkinson’s disease. Neuropsychobiology, 48(4), 190–193.
Wickremaratchi, M. M., Knipe, M. D., Sastry, B. S., Morgan, E., Jones, A., Salmon, R., et al. (2011). The motor phenotype of Parkinson’s disease in relation to age at onset. Movement Disorders, 26(3), 457–463.
Wilkins, R. C., & Lis, J. T. (1998). GAGA factor binding to DNA via a single trinucleotide sequence element. Nucleic Acids Research, 26(11), 2672e8.
Woo, N. H., Teng, H. K., Siao, C. J., Chiaruttini, C., Pang, P. T., Milner, T. A., et al. (2005). Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nature Neuroscience, 8(8), 1069–1077.
Wood, L. D. (2010). Clinical review and treatment of select adverse effects of dopamine receptor agonists in Parkinson’s disease. Drugs and Aging, 27(4), 295–310.
Xu, S., Liu, J., Yang, X., Qian, Y., & Xiao, Q. (2017). Association of the DRD2 CAn-STR and DRD3 Ser9Gly polymorphisms with Parkinson’s disease and response to dopamine agonists. Journal of the Neurological Sciences, 372, 433–438.
Yamada, H., Kuroki, T., Nakahara, T., Hashimoto, K., Tsutsumi, T., Hirano, M., et al. (2007). The dopamine D1 receptor agonist, but not the D2 receptor agonist, induces gene expression of Homer 1a in rat striatum and nucleus accumbens. Brain Research, 1131(1), 88–96.
Yamanaka, H., Nakajima, M., Katoh, M., Hara, Y., Tachibana, O., Yamashita, J., et al. (2004). A novel polymorphism in the promoter region of human UGT1A9 gene (UGT1A9*22) and its effects on the transcriptional activity. Pharmacogenetics, 14(5), 329–332.
Yin, B., Chen, Y., & Zhang, L. (2013). Association between catechol-O-methyltransferase (COMT) gene polymorphisms, Parkinson’s disease, and levodopa efficacy. Molecular Diagnosis and Therapy. https://doi.org/10.1007/s40291-013-0066-z.
Yu, L., Frith, M. C., Suzuki, Y., Peterfreund, R. A., Gearan, T., & Sugano, S. (2004). Characterization of genomic organization of the adenosine A2A receptor gene by molecular and bioinformatics analyses. Brain Research, 1000(1–2), 156–173.
Zahodne, L. B., & Fernandez, H. H. (2008). Pathophysiology and treatment of psychosis in Parkinson’s disease: A review. Drugs and Aging, 25(8), 665–682.
Zappia, M., Annesi, G., Nicoletti, G., Arabia, G., Annesi, F., Messina, D., et al. (2005). Sex differences in clinical and genetic determinants of levodopa peak-dose dyskinesias in Parkinson disease: An exploratory study. Archives of Neurology, 62(4), 601–605.
Zareparsi, S., Camicioli, R., Sexton, G., Bird, T., Swanson, P., Kaye, J., et al. (2002). Age at onset of Parkinson disease and Apolipoprotein E genotypes. American Journal of Medical Genetics, 107(2), 156–161.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Politi, C., Ciccacci, C., Novelli, G. et al. Genetics and Treatment Response in Parkinson’s Disease: An Update on Pharmacogenetic Studies. Neuromol Med 20, 1–17 (2018). https://doi.org/10.1007/s12017-017-8473-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-017-8473-7