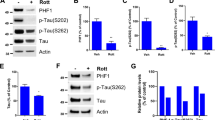
Abstract
The hyperphosphorylated tau is a major protein component of neurofibrillary tangle, which is one of hallmarks of Alzheimer’s disease (AD). While the level of methylglyoxal (MG) is significantly increased in the AD brains, the role of MG in tau phosphorylation is still not reported. Here, we found that MG could induce tau hyperphosphorylation at multiple AD-related sites in neuroblastoma 2a cells under maintaining normal cell viability. MG treatment increased the level of advanced glycation end products (AGEs) and the receptor of AGEs (RAGE). Glycogen synthesis kinase-3β (GSK-3β) and p38 MAPK were activated, whereas the level and activity of JNK, Erk1/2, cdk5, and PP2A were not altered after MG treatment. Simultaneous inhibition of GSK-3β or p38 attenuated the MG-induced tau hyperphosphorylation. Aminoguanidine, a blocker of AGEs formation, could effectively reverse the MG-induced tau hyperphosphorylation. These data suggest that MG induces AD-like tau hyperphosphorylation through AGEs formation involving RAGE up-regulation and GSK-3β activation and p38 activation is also partially involved in MG-induced tau hyperphosphorylation. Thus, targeting MG may be a promising therapeutic strategy to prevent AD-like tau hyperphosphorylation.







Similar content being viewed by others
References
Ahmed, M. U., Brinkmann, Frye. E., Degenhardt, T. P., Thorpe, S. R., & Baynes, J. W. (1997). N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. The Biochemical Journal, 324, 565–570.
Ahmed, N., & Thornalley, P. J. (2003). Quantitative screening of protein biomarkers of early glycation, advanced glycation, oxidation and nitrosation in cellular and extracellular proteins by tandem mass spectrometry multiple reaction monitoring. Biochemical Society Transactions, 31, 1417–1422.
Alafuzoff, I., Iqbal, K., Friden, H., Adolfsson, R., & Winblad, B. (1987). Histopathological criteria for progressive dementia disorders: Clinical-pathological correlation and classification by multivariate data analysis. Acta Neuropathologica, 74, 209–225.
Brownlee, M., Vlassara, H., Kooney, A., Ulrich, P., & Cerami, A. (1986). Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science, 232, 1629–1632.
Butterfield, D. A. (2002). Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity: Implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radical Research, 36, 1307–1313.
Cantero, A. V., Portero-Otin, M., Ayala, V., Auge, N., Sanson, M., Elbaz, M., et al. (2007). Methylglyoxal induces advanced glycation end product (AGEs) formation and dysfunction of PDGF receptor-beta: Implications for diabetic atherosclerosis. The FASEB Journal, 21, 3096–3106.
Chen F., Wollmer M. A., Hoerndli F., Munch G., Kuhla B., Rogaev E. I., Tsolaki M., Papassotiropoulos A., & Gotz J. (2004). Role for glyoxalase I in Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America, 101, 7687–7692.
de Arriba, S. G., Stuchbury, G., Yarin, J., Burnell, J., Loske, C., & Munch, G. (2007). Methylglyoxal impairs glucose metabolism and leads to energy depletion in neuronal cells–protection by carbonyl scavengers. Neurobiology of Aging, 28, 1044–1050.
Du, J., Suzuki, H., Nagase, F., Akhand, A. A., Ma, X. Y., Yokoyama, T., et al. (2001). Superoxide-mediated early oxidation and activation of ASK1 are important for initiating methylglyoxal-induced apoptosis process. Free Radical Biology and Medicine, 31, 469–478.
Duran-Jimenez, B., Dobler, D., Moffatt, S., Rabbani, N., Streuli, C. H., Thornalley, P. J., et al. (2009). Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes. Diabetes, 58, 2893–2903.
Fukunaga, M., Miyata, S., Liu, B. F., Miyazaki, H., Hirota, Y., Higo, S., et al. (2004). Methylglyoxal induces apoptosis through activation of p38 MAPK in rat Schwann cells. Biochemical Biophysical Research Communications, 320, 689–695.
Gong, C. X., Grundke-Iqbal, I., & Iqbal, K. (1994). Dephosphorylation of Alzheimer’s disease abnormally phosphorylated tau by protein phosphatase-2A. Neuroscience, 61, 765–772.
Grundke-Iqbal, I., Iqbal, K., Quinlan, M., Tung, Y. C., Zaidi, M. S., & Wisniewski, H. M. (1986). Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. The Journal of Biological Chemistry, 261, 6084–6089.
Guglielmotto, M., Aragno, M., Tamagno, E., Vercellinatto, I., Visentin, S., Medana, C., Catalano, M. G., Smith, M. A., Perry, G., Danni, O., Boccuzzi, G., & Tabaton, M. (2012). AGEs/RAGE complex upregulates BACE1 via NF-kappaB pathway activation. Neurobiology of Aging, 33, 196.e13–196.e27.
Hambsch, B., Chen, B. G., Brenndorfer, J., Meyer, M., Avrabos, C., Maccarrone, G., et al. (2010). Methylglyoxal-mediated anxiolysis involves increased protein modification and elevated expression of glyoxalase 1 in the brain. Journal of Neurochemistry, 113, 1240–1251.
Hu, M., Waring, J. F., Gopalakrishnan, M., & Li, J. (2008). Role of GSK-3beta activation and alpha7 nAChRs in Abeta(1–42)-induced tau phosphorylation in PC12 cells. Journal of Neurochemistry, 106, 1371–1377.
Iqbal, K., Alonso, Adel. C., Chen, S., Chohan, M. O., El-Akkad, E., Gong, C. X., et al. (2005). Tau pathology in Alzheimer disease and other tauopathies. Biochimica et Biophysica Acta, 1739, 198–210.
Iqbal, K., Liu, F., Gong, C. X., Alonso, A. C., & Grundke-Iqbal, I. (2009). Mechanisms of tau-induced neurodegeneration. Acta Neuropathologica, 118, 53–69.
Kopke, E., Tung, Y. C., Shaikh, S., Alonso, A. C., Iqbal, K., & Grundke-Iqbal, I. (1993). Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. The Journal of Biological Chemistry, 268, 24374–24384.
Kuhla, B., Boeck, K., Schmidt, A., Ogunlade, V., Arendt, T., Munch, G., et al. (2007). Age- and stage-dependent glyoxalase I expression and its activity in normal and Alzheimer’s disease brains. Neurobiology of Aging, 28, 29–41.
Kuhla, B., Luth, H. J., Haferburg, D., Boeck, K., Arendt, T., & Munch, G. (2005). Methylglyoxal, glyoxal, and their detoxification in Alzheimer’s disease. Annals of the New York Academy of Sciences, 1043, 211–216.
Kuhla, B., Luth, H. J., Haferburg, D., Weick, M., Reichenbach, A., Arendt, T., et al. (2006). Pathological effects of glyoxalase I inhibition in SH-SY5Y neuroblastoma cells. Journal of Neuroscience Research, 83, 1591–1600.
Li, X. H., Lv, B. L., Xie, J. Z., Liu, J., Zhou, X. W., & Wang, J. Z. (2012). AGEs induce Alzheimer-like tau pathology and memory deficit via RAGE-mediated GSK-3 activation. Neurobiology of Aging, 33, 1400–1410.
Liu, B. F., Miyata, S., Hirota, Y., Higo, S., Miyazaki, H., Fukunaga, M., et al. (2003). Methylglyoxal induces apoptosis through activation of p38 mitogen-activated protein kinase in rat mesangial cells. Kidney International, 63, 947–957.
Lo, T. W., Westwood, M. E., McLellan, A. C., Selwood, T., & Thornalley, P. J. (1994). Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. The Journal of Biological Chemistry, 269, 32299–32305.
Markesbery, W. R. (1997). Oxidative stress hypothesis in Alzheimer’s disease. Free Radical Biology and Medicine, 23, 134–147.
Perry, G., Castellani, R. J., Hirai, K., & Smith, M. A. (1998). Reactive oxygen species mediate cellular damage in alzheimer disease. Journal of Alzheimer’s Disease, 1, 45–55.
Phillips, S. A., & Thornalley, P. J. (1993). The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. European Journal of Biochemistry, 212, 101–105.
Planel, E., Miyasaka, T., Launey, T., Chui, D. H., Tanemura, K., Sato, S., et al. (2004). Alterations in glucose metabolism induce hypothermia leading to tau hyperphosphorylation through differential inhibition of kinase and phosphatase activities: Implications for Alzheimer’s disease. The Journal of Neuroscience, 24, 2401–2411.
Queisser, M. A., Yao, D., Geisler, S., Hammes, H. P., Lochnit, G., Schleicher, E. D., et al. (2010). Hyperglycemia impairs proteasome function by methylglyoxal. Diabetes, 59, 670–678.
Riboulet-Chavey, A., Pierron, A., Durand, I., Murdaca, J., Giudicelli, J., & Van Obberghen, E. (2006). Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes, 55, 1289–1299.
Richard, J. P. (1993). Mechanism for the formation of methylglyoxal from triosephosphates. Biochemical Society Transactions, 21, 549–553.
Rodrigues, L., Biasibetti, R., Swarowsky, A., Leite, M. C., Quincozes-Santos, A., Quilfeldt, J. A., et al. (2009). Hippocampal alterations in rats submitted to streptozotocin-induced dementia model are prevented by aminoguanidine. Journal of Alzheimer’s Disease, 17, 193–202.
Sasaki, N., Fukatsu, R., Tsuzuki, K., Hayashi, Y., Yoshida, T., Fujii, N., et al. (1998). Advanced glycation end products in Alzheimer’s disease and other neurodegenerative diseases. The American Journal of Pathology, 153, 1149–1155.
Schmidt, B., de Assis, A. M., Battu, C. E., Rieger, D. K., Hansen, F., Sordi, F., et al. (2010). Effects of glyoxal or methylglyoxal on the metabolism of amino acids, lactate, glucose and acetate in the cerebral cortex of young and adult rats. Brain Research, 1315, 19–24.
Shinohara, M., Thornalley, P. J., Giardino, I., Beisswenger, P., Thorpe, S. R., Onorato, J., et al. (1998). Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation end product formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. The Journal of Clinical Investigation, 101, 1142–1147.
Smith, M. A., Sayre, L. M., Anderson, V. E., Harris, P. L., Beal, M. F., Kowall, N., et al. (1998). Cytochemical demonstration of oxidative damage in Alzheimer disease by immunochemical enhancement of the carbonyl reaction with 2,4-dinitrophenylhydrazine. Journal of Histochemistry and Cytochemistry, 46, 731–735.
Soulis-Liparota, T., Cooper, M., Papazoglou, D., Clarke, B., & Jerums, G. (1991). Retardation by aminoguanidine of development of albuminuria, mesangial expansion, and tissue fluorescence in streptozocin-induced diabetic rat. Diabetes, 40, 1328–1334.
Srikanth, V., Maczurek, A., Phan, T., Steele, M., Westcott, B., Juskiw, D., et al. (2011). Advanced glycation end products and their receptor RAGE in Alzheimer’s disease. Neurobiology of Aging, 32, 763–777.
Thornalley, P. J. (2003). Glyoxalase I–structure, function and a critical role in the enzymatic defence against glycation. Biochemical Society Transactions, 31, 1343–1348.
Valente, T., Gella, A., Fernandez-Busquets, X., Unzeta, M., & Durany, N. (2010). Immunohistochemical analysis of human brain suggests pathological synergism of Alzheimer’s disease and diabetes mellitus. Neurobiology of Disease, 37, 67–76.
Vitek, M. P., Bhattacharya, K., Glendening, J. M., Stopa, E., Vlassara, H., Bucala, R., et al. (1994). Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America, 91, 4766–4770.
Vlassara, H., Fuh, H., Makita, Z., Krungkrai, S., Cerami, A., & Bucala, R. (1992). Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: a model for diabetic and aging complications. Proceedings of the National Academy of Sciences of the United States of America, 89, 12043–12047.
Vlassara, H., Striker, L. J., Teichberg, S., Fuh, H., Li, Y. M., & Steffes, M. (1994). Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proceedings of the National Academy of Sciences of the United States of America, 91, 11704–11708.
Wang, J. Z., Gong, C. X., Zaidi, T., Grundke-Iqbal, I., & Iqbal, K. (1995). Dephosphorylation of Alzheimer paired helical filaments by protein phosphatase-2A and -2B. The Journal of Biological Chemistry, 270, 4854–4860.
Wang, J. Z., & Liu, F. (2008). Microtubule-associated protein tau in development, degeneration and protection of neurons. Progress in Neurobiology, 85, 148–175.
Webster, J., Urban, C., Berbaum, K., Loske, C., Alpar, A., Gartner, U., et al. (2005). The carbonyl scavengers aminoguanidine and tenilsetam protect against the neurotoxic effects of methylglyoxal. Neurotoxicity Research, 7, 95–101.
Yamawaki, H., Saito, K., Okada, M., & Hara, Y. (2008). Methylglyoxal mediates vascular inflammation via JNK and p38 in human endothelial cells. American Journal of Physiology, 295, C1510–C1517.
Yan, S. D., Chen, X., Schmidt, A. M., Brett, J., Godman, G., Zou, Y. S., et al. (1994). Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proceedings of the National Academy of Sciences of the United States of America, 91, 7787–7791.
Acknowledgments
This work was supported by the National Nature Scientific Fund of China (No: 81171196).
Conflict of interest
There are no actual or potential conflicts of interest including any financial, personal, or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence (bias) our work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, XH., Xie, JZ., Jiang, X. et al. Methylglyoxal Induces Tau Hyperphosphorylation via Promoting AGEs Formation. Neuromol Med 14, 338–348 (2012). https://doi.org/10.1007/s12017-012-8191-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-012-8191-0