Abstract
Duchenne Muscular Dystrophy (DMD) is an inherited genetic disorder characterized by progressive degeneration of muscle tissue, leading to functional disability and premature death. Despite extensive research efforts, the discovery of a cure for DMD continues to be elusive, emphasizing the need to investigate novel treatment approaches. Cellular therapies have emerged as prospective approaches to address the underlying pathophysiology of DMD. This review provides an examination of the present situation regarding cell-based therapies, including CD133 + cells, muscle precursor cells, mesoangioblasts, bone marrow-derived mononuclear cells, mesenchymal stem cells, cardiosphere-derived cells, and dystrophin-expressing chimeric cells. A total of 12 studies were found eligible to be included as they were completed cell therapy clinical trials, clinical applications, or case reports with quantitative results. The evaluation encompassed an examination of limitations and potential advancements in this particular area of research, along with an assessment of the safety and effectiveness of cell-based therapies in the context of DMD. In general, the available data indicates that diverse cell therapy approaches may present a new, safe, and efficacious treatment modality for patients diagnosed with DMD. However, further studies are required to comprehensively understand the most advantageous treatment approach and therapeutic capacity.
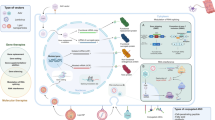
Graphical Abstract



Similar content being viewed by others
Availability of Data and Material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mercuri, E., & Muntoni, F. (2013). Muscular dystrophies. The Lancet, 381(9869), 845–860. https://doi.org/10.1016/s0140-6736(12)61897-2
Emery, A. E. (2002). The muscular dystrophies. The Lancet, 359(9307), 687–695. https://doi.org/10.1016/s0140-6736(02)07815-7
Connuck, D. M., Sleeper, L. A., Colan, S. D., Cox, G. F., Towbin, J. A., Lowe, A. M., Wilkinson, J. D., Orav, E. J., Cuniberti, L., Salbert, B. A., Lipshultz, S. E., Pediatric Cardiomyopathy Registry Study Group. (2008). Characteristics and outcomes of cardiomyopathy in children with Duchenne or Becker muscular dystrophy: a comparative study from the Pediatric Cardiomyopathy Registry. American Heart Journal, 155(6), 998–1005. https://doi.org/10.1016/j.ahj.2008.01.018
Freilinger, M., Schmidt, I., Dysek, S., Seidl, R., Schmidt, W. M., & Bittner, R. E. (2013). Muscle-specific discordant skewing of X chromosome inactivation leads to different clinical phenotypes of Duchenne muscular dystrophy in two monozygotic female twins. Neuropediatrics, 44, FV16_04.
Ferrier, P., Bamatter, F., & Klein, D. (1965). Muscular dystrophy (Duchenne) in a girl with Turner’s syndrome. Journal of Medical Genetics, 2(1), 38–46. https://doi.org/10.1136/jmg.2.1.38
Chelly, J., Marlhens, F., Le Marec, B., Jeanpierre, M., Lambert, M., Hamard, G., Dutrillaux, B., & Kaplan, J. C. (1986). De novo DNA microdeletion in a girl with Turner syndrome and Duchenne muscular dystrophy. Human Genetics, 74(2), 193–196. https://doi.org/10.1007/BF00282093
Satre, V., Monnier, N., Devillard, F., Amblard, F., & Lunardi, J. (2004). Prenatal diagnosis of DMD in a female fetus affected by Turner syndrome. Prenatal Diagnosis, 24(11), 913–917. https://doi.org/10.1002/pd.1031
Birnkrant, D. J., Bushby, K., Bann, C. M., Apkon, S. D., Blackwell, A., Brumbaugh, D., Case, L. E., Clemens, P. R., Hadjiyannakis, S., Pandya, S., Street, N., Tomezsko, J., Wagner, K. R., Ward, L. M., Weber, D. R., DMD Care Considerations Working Group. (2018). Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. The Lancet. Neurology, 17(3), 251–267. https://doi.org/10.1016/S1474-4422(18)30024-3
Patterson, G., Conner, H., Groneman, M., Blavo, C., & Parmar, M. S. (2023). Duchenne muscular dystrophy: Current treatment and emerging exon skipping and gene therapy approach. European Journal of Pharmacology, 947, 175675. https://doi.org/10.1016/j.ejphar.2023.175675
Deconinck, N., & Dan, B. (2007). Pathophysiology of Duchenne muscular dystrophy: Current hypotheses. Pediatric Neurology, 36(1), 1–7. https://doi.org/10.1016/j.pediatrneurol.2006.09.016
Duan, D., Goemans, N., Takeda, S., Mercuri, E., & Aartsma-Rus, A. (2021). Duchenne muscular dystrophy. Nature Reviews Disease Primers, 7(1), 13. https://doi.org/10.1038/s41572-021-00248-3
Thangarajh, M., Hendriksen, J., McDermott, M. P., Martens, W., Hart, K. A., Griggs, R. C., Muscle Study Group and TREAT-NMD. (2019). Relationships between DMD mutations and neurodevelopment in dystrophinopathy. Neurology, 93(17), e1597–e1604. https://doi.org/10.1212/WNL.0000000000008363
Moat, S. J., Bradley, D. M., Salmon, R., Clarke, A., & Hartley, L. (2013). Newborn bloodspot screening for Duchenne muscular dystrophy: 21 years experience in Wales (UK). European Journal of Human Genetics, 21(10), 1049–1053. https://doi.org/10.1038/ejhg.2012.301
Mendell, J. R., Sahenk, Z., Lehman, K., Nease, C., Lowes, L. P., Miller, N. F., Iammarino, M. A., Alfano, L. N., Nicholl, A., Al-Zaidy, S., Lewis, S., Church, K., Shell, R., Cripe, L. H., Potter, R. A., Griffin, D. A., Pozsgai, E., Dugar, A., Hogan, M., & Rodino-Klapac, L. R. (2020). Assessment of Systemic Delivery of rAAVrh74.MHCK7.micro-dystrophin in Children With Duchenne Muscular Dystrophy: A Nonrandomized Controlled Trial. JAMA Neurology, 77(9), 1122–1131. https://doi.org/10.1001/jamaneurol.2020.1484
Bellinger, A. M., Reiken, S., Carlson, C., Mongillo, M., Liu, X., Rothman, L., Matecki, S., Lacampagne, A., & Marks, A. R. (2009). Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nature Medicine, 15(3), 325–330. https://doi.org/10.1038/nm.1916
Passamano, L., Taglia, A., Palladino, A., Viggiano, E., D’Ambrosio, P., Scutifero, M., Rosaria Cecio, M., Torre, V., De Luca, F., Picillo, E., Paciello, O., Piluso, G., Nigro, G., & Politano, L. (2012). Improvement of survival in Duchenne Muscular Dystrophy: retrospective analysis of 835 patients. Acta Myologica : Myopathies and Cardiomyopathies: Official Journal of the Mediterranean Society of Myology, 31(2), 121–125.
Lechner, A., Herzig, J. J., Kientsch, J. G., Kohler, M., Bloch, K. E., Ulrich, S., & Schwarz, E. I. (2023). Cardiomyopathy as cause of death in Duchenne muscular dystrophy: A longitudinal observational study. ERJ Open Research, 9(5), 00176–02023. https://doi.org/10.1183/23120541.00176-2023
Mendell, J. R., Shilling, C., Leslie, N. D., Flanigan, K. M., al-Dahhak, R., Gastier-Foster, J., Kneile, K., Dunn, D. M., Duval, B., Aoyagi, A., Hamil, C., Mahmoud, M., Roush, K., Bird, L., Rankin, C., Lilly, H., Street, N., Chandrasekar, R., & Weiss, R. B. (2012). Evidence-based path to newborn screening for Duchenne muscular dystrophy. Annals of Neurology, 71(3), 304–313. https://doi.org/10.1002/ana.23528
Romitti, P. A., Zhu, Y., Puzhankara, S., James, K. A., Nabukera, S. K., Zamba, G. K., Ciafaloni, E., Cunniff, C., Druschel, C. M., Mathews, K. D., Matthews, D. J., Meaney, F. J., Andrews, J. G., Conway, K. M., Fox, D. J., Street, N., Adams, M. M., Bolen, J., MD STARnet. (2015). Prevalence of Duchenne and Becker muscular dystrophies in the United States. Pediatrics, 135(3), 513–521. https://doi.org/10.1542/peds.2014-2044
Deng, J., Zhang, J., Shi, K., & Liu, Z. (2022). Drug development progress in Duchenne muscular dystrophy. Frontiers in Pharmacology, 13, 950651. https://doi.org/10.3389/fphar.2022.950651
Lim, K. R., Maruyama, R., & Yokota, T. (2017). Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Design, Development and Therapy, 11, 533–545. https://doi.org/10.2147/DDDT.S97635
Nakamura, A., Shiba, N., Miyazaki, D., Nishizawa, H., Inaba, Y., Fueki, N., Maruyama, R., Echigoya, Y., & Yokota, T. (2017). Comparison of the phenotypes of patients harboring in-frame deletions starting at exon 45 in the Duchenne muscular dystrophy gene indicates potential for the development of exon skipping therapy. Journal of Human Genetics, 62(4), 459–463. https://doi.org/10.1038/jhg.2016.152
Sun, C., Serra, C., Lee, G., & Wagner, K. R. (2020). Stem cell-based therapies for Duchenne muscular dystrophy. Experimental Neurology, 323, 113086. https://doi.org/10.1016/j.expneurol.2019.113086
Law, P. K., Goodwin, T. G., Fang, Q. W., Chen, M., Li, H. J., Florendo, J. A., & Kirby, D. S. (1991). Myoblast transfer therapy for Duchenne muscular dystrophy. Acta Paediatrica Japonica, 33(2), 206–215. https://doi.org/10.1111/j.1442-200x.1991.tb01545.x
Law, P. K., Goodwin, T. G., Fang, Q., Duggirala, V., Larkin, C., Florendo, J. A., Kirby, D. S., Deering, M. B., Li, H. J., & Chen, M. (1992). Feasibility, safety, and efficacy of myoblast transfer therapy on Duchenne muscular dystrophy boys. Cell Transplantation, 1(2–3), 235–244. https://doi.org/10.1177/0963689792001002-305
Huard, J., Bouchard, J. P., Roy, R., Malouin, F., Dansereau, G., Labrecque, C., Albert, N., Richards, C. L., Lemieux, B., & Tremblay, J. P. (1992). Human myoblast transplantation: Preliminary results of 4 cases. Muscle & Nerve, 15(5), 550–560. https://doi.org/10.1002/mus.880150504
Gussoni, E., Pavlath, G. K., Lanctot, A. M., Sharma, K. R., Miller, R. G., Steinman, L., & Blau, H. M. (1992). Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature, 356(6368), 435–438. https://doi.org/10.1038/356435a0
Tremblay, J. P., Bouchard, J. P., Malouin, F., Théau, D., Cottrell, F., Collin, H., Rouche, A., Gilgenkrantz, S., Abbadi, N., & Tremblay, M. (1993). Myoblast transplantation between monozygotic twin girl carriers of Duchenne muscular dystrophy. Neuromuscular Disorders: NMD, 3(5–6), 583–592. https://doi.org/10.1016/0960-8966(93)90121-y
Hogrel, J. Y., Zagnoli, F., Canal, A., Fraysse, B., Bouchard, J. P., Skuk, D., Fardeau, M., & Tremblay, J. P. (2013). Assessment of a symptomatic Duchenne muscular dystrophy carrier 20 years after myoblast transplantation from her asymptomatic identical twin sister. Neuromuscular Disorders: NMD, 23(7), 575–579. https://doi.org/10.1016/j.nmd.2013.04.007
Karpati, G., Ajdukovic, D., Arnold, D., Gledhill, R. B., Guttmann, R., Holland, P., Koch, P. A., Shoubridge, E., Spence, D., & Vanasse, M. (1993). Myoblast transfer in Duchenne muscular dystrophy. Annals of Neurology, 34(1), 8–17. https://doi.org/10.1002/ana.410340105
Mendell, J. R., Kissel, J. T., Amato, A. A., King, W., Signore, L., Prior, T. W., Sahenk, Z., Benson, S., McAndrew, P. E., & Rice, R. (1995). Myoblast transfer in the treatment of Duchenne’s muscular dystrophy. The New England Journal of Medicine, 333(13), 832–838. https://doi.org/10.1056/NEJM199509283331303
Miller, R. G., Sharma, K. R., Pavlath, G. K., Gussoni, E., Mynhier, M., Lanctot, A. M., Greco, C. M., Steinman, L., & Blau, H. M. (1997). Myoblast implantation in Duchenne muscular dystrophy: The San Francisco study. Muscle & Nerve, 20(4), 469–478. https://doi.org/10.1002/(sici)1097-4598(199704)20:4%3c469::aid-mus10%3e3.0.co;2-u
Skuk, D., Goulet, M., Roy, B., Piette, V., Côté, C. H., Chapdelaine, P., Hogrel, J., Paradis, M., Bouchard, J. P., Sylvain, M., Lachance, G., & Tremblay, J. P. (2007). First test of a “high-density injection” protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: Eighteen months follow-up. Neuromuscular Disorders, 17(1), 38–46. https://doi.org/10.1016/j.nmd.2006.10.003
Torrente, Y., Belicchi, M., Marchesi, C., D’Antona, G., Cogiamanian, F., Pisati, F., Gavina, M., Giordano, R., Tonlorenzi, R., Fagiolari, G., Lamperti, C., Porretti, L., Lopa, R., Sampaolesi, M., Vicentini, L., Grimoldi, N., Tiberio, F., Songa, V., Baratta, P., … Bresolin, N. (2007). Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell Transplantation, 16(6), 563–577. https://doi.org/10.3727/000000007783465064
Sharma, A., Sane, H., Badhe, P., Gokulchandran, N., Kulkarni, P., Lohiya, M., Biju, H., & Jacob, V. C. (2013). A clinical study shows safety and efficacy of autologous bone marrow mononuclear cell therapy to improve quality of life in muscular dystrophy patients. Cell Transplantation, 22(Suppl 1), S127–S138. https://doi.org/10.3727/096368913X672136
Sharma, A., Sane, H., Paranjape, A., Bhagawanani, K., Gokulchandran, N., & Badhe, P. (2014). Autologous bone marrow mononuclear cell transplantation in Duchenne muscular dystrophy - a case report. The American Journal of Case Reports, 15, 128–134. https://doi.org/10.12659/AJCR.890078
Cossu, G., Previtali, S. C., Napolitano, S., Cicalese, M. P., Tedesco, F. S., Nicastro, F., Noviello, M., Roostalu, U., Natali Sora, M. G., Scarlato, M., De Pellegrin, M., Godi, C., Giuliani, S., Ciotti, F., Tonlorenzi, R., Lorenzetti, I., Rivellini, C., Benedetti, S., Gatti, R., Marktel, S., … Ciceri, F. (2015). Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy. EMBO Molecular Medicine, 7(12), 1513–1528. https://doi.org/10.15252/emmm.201505636
Rajput, B. S., Chakrabarti, S. K., Dongare, V. S., Ramirez, C. M., & Deb, K. D. (2015). Human Umbilical Cord Mesenchymal Stem Cells in the Treatment of Duchenne Muscular Dystrophy: Safety and Feasibility Study in India. Journal of Stem Cells, 10(2), 141–156.
Dai, A., Baspinar, O., Yeşilyurt, A., Sun, E., Aydemir, Ç. İ, Öztel, O. N., Capkan, D. U., Pinarli, F., Agar, A., & Karaöz, E. (2018). Efficacy of stem cell therapy in ambulatory and nonambulatory children with Duchenne muscular dystrophy - Phase I-II. Degenerative Neurological and Neuromuscular Disease, 8, 63–77. https://doi.org/10.2147/DNND.S170087
Rajput, B., Meher, K., Somalapur, P., Kulkarni, R., Bopardikar, A., & Kumar, R. (2019). Safety and Efficacy of a Combination Therapy (Autologous Bone Marrow-Derived Mononuclear Cells and Umbilical Cord-Derived Mesenchymal Stem Cells) in Duchenne Muscular Dystrophy Patients. Journal of Embryology & Stem Cell Research, 3(2). https://doi.org/10.23880/jes-16000127.
Świątkowska-Flis, B., Zdolińska-Malinowska, I., Sługocka, D., & Boruczkowski, D. (2021). The use of umbilical cord-derived mesenchymal stem cells in patients with muscular dystrophies: Results from compassionate use in real-life settings. Stem Cells Translational Medicine, 10(10), 1372–1383. https://doi.org/10.1002/sctm.21-0027
Taylor, M., Jefferies, J., Byrne, B., Lima, J., Ambale-Venkatesh, B., Ostovaneh, M. R., Makkar, R., Goldstein, B., Smith, R. R., Fudge, J., Malliaras, K., Fedor, B., Rudy, J., Pogoda, J. M., Marbán, L., Ascheim, D. D., Marbán, E., & Victor, R. G. (2019). Cardiac and skeletal muscle effects in the randomized HOPE-Duchenne trial. Neurology, 92(8), e866–e878. https://doi.org/10.1212/WNL.0000000000006950
McDonald, C. M., Marbán, E., Hendrix, S., Hogan, N., Ruckdeschel Smith, R., Eagle, M., Finkel, R. S., Tian, C., Janas, J., Harmelink, M. M., Varadhachary, A. S., Taylor, M. D., Hor, K. N., Mayer, O. H., Henricson, E. K., Furlong, P., Ascheim, D. D., Rogy, S., Williams, P., Marbán, L., … HOPE-2 Study Group (2022). Repeated intravenous cardiosphere-derived cell therapy in late-stage Duchenne muscular dystrophy (HOPE-2): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet (London, England), 399(10329), 1049–1058. https://doi.org/10.1016/S0140-6736(22)00012-5
Heydemann, A., Bieganski, G., Wachowiak, J., Czarnota, J., Niezgoda, A., Siemionow, K., Ziemiecka, A., Sikorska, M. H., Bozyk, K., Tullius, S. G., & Siemionow, M. (2023). Dystrophin Expressing Chimeric (DEC) Cell Therapy for Duchenne Muscular Dystrophy: A First-in-Human Study with Minimum 6 Months Follow-up. Stem Cell Reviews and Reports, 19(5), 1340–1359. https://doi.org/10.1007/s12015-023-10530-4
Siemionow, M., Biegański, G., Niezgoda, A., Wachowiak, J., Czarnota, J., Siemionow, K., Ziemiecka, A., Sikorska, M. H., Bożyk, K., & Heydemann, A. (2023). Safety and Efficacy of DT-DEC01 Therapy in Duchenne Muscular Dystrophy Patients: A 12 - Month Follow-Up Study After Systemic Intraosseous Administration. Stem Cell Reviews and Reports. https://doi.org/10.1007/s12015-023-10620-3.Advanceonlinepublication.10.1007/s12015-023-10620-3
Negroni, E., Riederer, I., Chaouch, S., Belicchi, M., Razini, P., Di Santo, J., Torrente, Y., Butler-Browne, G. S., & Mouly, V. (2009). In vivo myogenic potential of human CD133+ muscle-derived stem cells: A quantitative study. Molecular Therapy: The Journal of the American Society of Gene Therapy, 17(10), 1771–1778. https://doi.org/10.1038/mt.2009.167
Meng, J., Chun, S., Asfahani, R., Lochmüller, H., Muntoni, F., & Morgan, J. (2014). Human skeletal muscle-derived CD133+ cells form functional satellite cells after intramuscular transplantation in immunodeficient host mice. Molecular Therapy, 22(5), 1008–1017. https://doi.org/10.1038/mt.2014.26
Bittner, R. E., Schöfer, C., Weipoltshammer, K., Ivanova, S., Streubel, B., Hauser, E., Freilinger, M., Höger, H., Elbe-Bürger, A., & Wachtler, F. (1999). Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anatomy and Embryology, 199(5), 391–396. https://doi.org/10.1007/s004290050237
Nygren, J. M., Liuba, K., Breitbach, M., Stott, S., Thorén, L., Roell, W., Geisen, C., Sasse, P., Kirik, D., Björklund, A., Nerlov, C., Fleischmann, B. K., Jovinge, S., & Jacobsen, S. E. (2008). Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion. Nature Cell Biology, 10(5), 584–592. https://doi.org/10.1038/ncb1721
Sharma, A., Gokulchandran, N., Chopra, G., Kulkarni, P., Lohia, M., Badhe, P., & Jacob, V. C. (2012). Administration of autologous bone marrow-derived mononuclear cells in children with incurable neurological disorders and injury is safe and improves their quality of life. Cell Transplantation, 21(Suppl 1), S79–S90. https://doi.org/10.3727/096368912X633798
Yang, X. F., Xu, Y. F., Zhang, Y. B., Wang, H. M., Lü, N. W., Wu, Y. X., Lü, X., Cui, J. P., Shan, H., Yan, Y., & Zhou, J. X. (2009). Functional improvement of patients with progressive muscular dystrophy by bone marrow and umbilical cord blood mesenchymal stem cell transplantations. Zhonghua Yi Xue Za Zhi, 89(36), 2552–2556.
Minasi, M. G., Riminucci, M., De Angelis, L., Borello, U., Berarducci, B., Innocenzi, A., Caprioli, A., Sirabella, D., Baiocchi, M., De Maria, R., Boratto, R., Jaffredo, T., Broccoli, V., Bianco, P., & Cossu, G. (2002). The meso-angioblast: A multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development (Cambridge, England), 129(11), 2773–2783. https://doi.org/10.1242/dev.129.11.2773
Sampaolesi, M., & Cossu, G. (2009). Chapter 60 - Stem Cells for the Treatment of Muscular Dystrophy: Therapeutic Perspectives. In R. Lanza, J. Gearhart, B. Hogan, D. Melton, R. Pedersen, E. D. Thomas, J. Thomson, & I. Wilmut (Eds.), Essentials of Stem Cell Biology (2nd ed., pp. 543–550). Academic Press.
Caplan, A. I. (2007). Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. Journal of Cellular Physiology, 213(2), 341–347. https://doi.org/10.1002/jcp.21200
Elhussieny, A., Nogami, K., Sakai-Takemura, F., Maruyama, Y., Omar Abdelbakey, A., Abou El-kheir, W., Takeda, S., & Miyagoe-Suzuki, Y. (2020). Mesenchymal Stem Cells for Regenerative Medicine for Duchenne Muscular Dystrophy. IntechOpen. https://doi.org/10.5772/intechopen.92824
Kern, S., Eichler, H., Stoeve, J., Klüter, H., & Bieback, K. (2006). Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells, 24(5), 1294–1301. https://doi.org/10.1634/stemcells.2005-0342
Wang, Q., Yang, Q., Wang, Z., Tong, H., Ma, L., Zhang, Y., Shan, F., Meng, Y., & Yuan, Z. (2016). Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton’s jelly as sources of cell immunomodulatory therapy. Human Vaccines & Immunotherapeutics, 12(1), 85–96. https://doi.org/10.1080/21645515.2015.1030549
Kwon, S., Ki, S. M., Park, S. E., Kim, M. J., Hyung, B., Lee, N. K., Shim, S., Choi, B. O., Na, D. L., Lee, J. E., & Chang, J. W. (2016). Anti-apoptotic Effects of Human Wharton’s Jelly-derived Mesenchymal Stem Cells on Skeletal Muscle Cells Mediated via Secretion of XCL1. Molecular Therapy: The Journal of the American Society of Gene Therapy, 24(9), 1550–1560. https://doi.org/10.1038/mt.2016.125
Rogers, R. G., Fournier, M., Sanchez, L., Ibrahim, A. G., Aminzadeh, M. A., Lewis, M. I., & Marbán, E. (2019). Disease-modifying bioactivity of intravenous cardiosphere-derived cells and exosomes in mdx mice. JCI Insight, 4(7), e125754. https://doi.org/10.1172/jci.insight.125754
Marbán, E. (2018). A mechanistic roadmap for the clinical application of cardiac cell therapies. Nature Biomedical Engineering, 2, 353–361. https://doi.org/10.1038/s41551-018-0216-z
Makkar, R. R., Kereiakes, D. J., Aguirre, F., Kowalchuk, G., Chakravarty, T., Malliaras, K., Francis, G. S., Povsic, T. J., Schatz, R., Traverse, J. H., Pogoda, J. M., Smith, R. R., Marbán, L., Ascheim, D. D., Ostovaneh, M. R., Lima, J. A. C., DeMaria, A., Marbán, E., & Henry, T. D. (2020). Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): A randomized, placebo-controlled, double-blinded trial. European Heart Journal, 41(36), 3451–3458. https://doi.org/10.1093/eurheartj/ehaa541
Chimenti, I., Smith, R. R., Li, T. S., Gerstenblith, G., Messina, E., Giacomello, A., & Marbán, E. (2010). Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circulation Research, 106(5), 971–980. https://doi.org/10.1161/CIRCRESAHA.109.210682
Ibrahim, A. G., Cheng, K., & Marbán, E. (2014). Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports, 2(5), 606–619. https://doi.org/10.1016/j.stemcr.2014.04.006
Aminzadeh, M. A., Rogers, R. G., Fournier, M., Tobin, R. E., Guan, X., Childers, M. K., Andres, A. M., Taylor, D. J., Ibrahim, A., Ding, X., Torrente, A., Goldhaber, J. M., Lewis, M., Gottlieb, R. A., Victor, R. A., & Marbán, E. (2018). Exosome-Mediated Benefits of Cell Therapy in Mouse and Human Models of Duchenne Muscular Dystrophy. Stem Cell Reports, 10(3), 942–955. https://doi.org/10.1016/j.stemcr.2018.01.023
Sienkiewicz, D., Kulak, W., Okurowska-Zawada, B., & Paszko-Patej, G. (2015). Duchenne muscular dystrophy: Current cell therapies. Therapeutic Advances in Neurological Disorders, 8(4), 166–177. https://doi.org/10.1177/1756285615586123
Noviello, M., Tedesco, F. S., Bondanza, A., Tonlorenzi, R., Rosaria Carbone, M., Gerli, M. F. M., Marktel, S., Napolitano, S., Cicalese, M. P., Ciceri, F., Peretti, G., Cossu, G., & Bonini, C. (2014). Inflammation converts human mesoangioblasts into targets of alloreactive immune responses: Implications for allogeneic cell therapy of DMD. Molecular Therapy: The Journal of the American Society of Gene Therapy, 22(7), 1342–1352. https://doi.org/10.1038/mt.2014.62
Siemionow, M., Demir, Y., Mukherjee, A., & Klimczak, A. (2005). Development and maintenance of donor-specific chimerism in semi-allogenic and fully major histocompatibility complex mismatched facial allograft transplants. Transplantation, 79(5), 558–567. https://doi.org/10.1097/01.tp.0000152799.16035.b7
Siemionow, M., Klimczak, A., Unal, S., Agaoglu, G., & Carnevale, K. (2008). Hematopoietic stem cell engraftment and seeding permits multi-lymphoid chimerism in vascularized bone marrow transplants. American Journal of Transplantation, 8(6), 1163–1176. https://doi.org/10.1111/j.1600-6143.2008.02241.x
Siemionow, M., Cwykiel, J., Heydemann, A., Garcia-Martinez, J., Siemionow, K., & Szilagyi, E. (2018). Creation of Dystrophin Expressing Chimeric Cells of Myoblast Origin as a Novel Stem Cell Based Therapy for Duchenne Muscular Dystrophy. Stem Cell Reviews and Reports, 14(2), 189–199. https://doi.org/10.1007/s12015-017-9792-7
Siemionow, M., Langa, P., Harasymczuk, M., Cwykiel, J., Sielewicz, M., Smieszek, J., & Heydemann, A. (2021). Human dystrophin expressing chimeric (DEC) cell therapy ameliorates cardiac, respiratory, and skeletal muscle’s function in Duchenne muscular dystrophy. Stem Cells Translational Medicine, 10(10), 1406–1418. https://doi.org/10.1002/sctm.21-0054
Hotta, A. (2015). Genome Editing Gene Therapy for Duchenne Muscular Dystrophy. Journal of Neuromuscular Diseases, 2(4), 343–355. https://doi.org/10.3233/JND-150116
Elangkovan, N., & Dickson, G. (2021). Gene Therapy for Duchenne Muscular Dystrophy. Journal of Neuromuscular Diseases, 8(s2), S303–S316. https://doi.org/10.3233/JND-210678
Kapsa, R., Kornberg, A. J., & Byrne, E. (2003). Novel therapies for Duchenne muscular dystrophy. The Lancet Neurology, 2(5), 299–310. https://doi.org/10.1016/s1474-4422(03)00382-x
Ciafaloni, E., & Moxley, R. T. (2008). Treatment options for Duchenne muscular dystrophy. Current Treatment Options in Neurology, 10(2), 86–93. https://doi.org/10.1007/s11940-008-0010-4
Iftikhar, M., Frey, J., Shohan, M. J., Malek, S., & Mousa, S. A. (2021). Current and emerging therapies for Duchenne muscular dystrophy and spinal muscular atrophy. Pharmacology & Therapeutics, 220, 107719. https://doi.org/10.1016/j.pharmthera.2020.107719
Kinoshita, I., Vilquin, J. T., Guérette, B., Asselin, I., Roy, R., & Tremblay, J. P. (1994). Very efficient myoblast allotransplantation in mice under FK506 immunosuppression. Muscle & Nerve, 17(12), 1407–1415. https://doi.org/10.1002/mus.880171210
Kinoshita, I., Roy, R., Dugré, F. J., Gravel, C., Roy, B., Goulet, M., Asselin, I., & Tremblay, J. P. (1996). Myoblast transplantation in monkeys: Control of immune response by FK506. Journal of Neuropathology and Experimental Neurology, 55(6), 687–697. https://doi.org/10.1097/00005072-199606000-00002
Skuk, D., Roy, B., Goulet, M., Chapdelaine, P., Bouchard, J. P., Roy, R., Dugré, F. J., Lachance, J. G., Deschênes, L., Hélène, S., Sylvain, M., & Tremblay, J. P. (2004). Dystrophin expression in myofibers of Duchenne muscular dystrophy patients following intramuscular injections of normal myogenic cells. Molecular Therapy : The Journal of the American Society of Gene Therapy, 9(3), 475–482. https://doi.org/10.1016/j.ymthe.2003.11.023
Skuk, D., Goulet, M., Roy, B., Chapdelaine, P., Bouchard, J. P., Roy, R., Dugré, F. J., Sylvain, M., Lachance, J. G., Deschênes, L., Senay, H., & Tremblay, J. P. (2006). Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. Journal of Neuropathology and Experimental Neurology, 65(4), 371–386. https://doi.org/10.1097/01.jnen.0000218443.45782.81
Tremblay, J. P., Skuk, D., Palmieri, B., & Rothstein, D. M. (2009). A case for immunosuppression for myoblast transplantation in Duchenne muscular dystrophy. Molecular Therapy, 17(7), 1122–1124. https://doi.org/10.1038/mt.2009.125
Quenneville, S. P., Chapdelaine, P., Rousseau, J., Beaulieu, J., Caron, N. J., Skuk, D., Mills, P., Olivares, E. C., Calos, M. P., & Tremblay, J. P. (2004). Nucleofection of muscle-derived stem cells and myoblasts with phiC31 integrase: Stable expression of a full-length-dystrophin fusion gene by human myoblasts. Molecular Therapy : The Journal of the American Society of Gene Therapy, 10(4), 679–687. https://doi.org/10.1016/j.ymthe.2004.05.034
Li, S., Kimura, E., Fall, B. M., Reyes, M., Angello, J. C., Welikson, R., Hauschka, S. D., & Chamberlain, J. S. (2005). Stable transduction of myogenic cells with lentiviral vectors expressing a minidystrophin. Gene Therapy, 12(14), 1099–1108. https://doi.org/10.1038/sj.gt.3302505
Quenneville, S. P., Chapdelaine, P., Skuk, D., Paradis, M., Goulet, M., Rousseau, J., Xiao, X., Garcia, L., & Tremblay, J. P. (2007). Autologous transplantation of muscle precursor cells modified with a lentivirus for muscular dystrophy: Human cells and primate models. Molecular Therapy: The Journal of the American Society of Gene Therapy, 15(2), 431–438. https://doi.org/10.1038/sj.mt.6300047
Meng, J., Muntoni, F., & Morgan, J. (2018). CD133+ cells derived from skeletal muscles of Duchenne muscular dystrophy patients have a compromised myogenic and muscle regenerative capability. Stem Cell Research, 30, 43–52. https://doi.org/10.1016/j.scr.2018.05.004
Sanchez-Ramos, J. R. (2002). Neural cells derived from adult bone marrow and umbilical cord blood. Journal of Neuroscience Research, 69(6), 880–893. https://doi.org/10.1002/jnr.10337
LaBarge, M. A., & Blau, H. M. (2002). Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell, 111(4), 589–601. https://doi.org/10.1016/s0092-8674(02)01078-4
Price, F. D., Kuroda, K., & Rudnicki, M. A. (2007). Stem cell based therapies to treat muscular dystrophy. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1772(2), 272–283. https://doi.org/10.1016/j.bbadis.2006.08.011
Cuende, N., Rico, L., & Herrera, C. (2012). Concise review: Bone marrow mononuclear cells for the treatment of ischemic syndromes: Medicinal product or cell transplantation? Stem Cells Translational Medicine, 1(5), 403–408. https://doi.org/10.5966/sctm.2011-0064
Sharma, A., Sane, H., Gokulchandra, N., Sharan, R., Paranjape, A., Kulkarni, P., Yadav, J., & Badhe, P. (2016). Effect of Cellular Therapy in Progression of Becker’s Muscular Dystrophy: A Case Study. European Journal of Translational Myology, 26(1), 5522. https://doi.org/10.4081/ejtm.2016.5522
Burdon, T. J., Paul, A., Noiseux, N., Prakash, S., & Shum-Tim, D. (2011). Bone marrow stem cell derived paracrine factors for regenerative medicine: Current perspectives and therapeutic potential. Bone Marrow Research, 2011, 207326. https://doi.org/10.1155/2011/207326
Quattrocelli, M., Palazzolo, G., Perini, I., Crippa, S., Cassano, M., & Sampaolesi, M. (2012). Mouse and human mesoangioblasts: Isolation and characterization from adult skeletal muscles. Methods in Molecular Biology, 798, 65–76. https://doi.org/10.1007/978-1-61779-343-1_4
Bonfanti, C., Rossi, G., Tedesco, F. S., Giannotta, M., Benedetti, S., Tonlorenzi, R., Antonini, S., Marazzi, G., Dejana, E., Sassoon, D., Cossu, G., & Messina, G. (2015). PW1/Peg3 expression regulates key properties that determine mesoangioblast stem cell competence. Nature Communications, 6, 6364. https://doi.org/10.1038/ncomms7364
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AA: Conceived and designed the analysis, collected data, analyzed results, drafted the article. EK: Study conception and design, revised the article critically for important intellectual content.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent to Participate
Both authors hereby provide our consent to participate in the submission of the manuscript titled "Cell Therapy Strategies for Duchenne Muscular Dystrophy: A Systematic Review of Clinical Applications" to the Stem Cell Review and Reports Journal. We understand and agree to the following terms and conditions:
We confirm that we have read, reviewed, and contributed to the creation of the manuscript titled "Cell Therapy Strategies for Duchenne Muscular Dystrophy: A Systematic Review of Clinical Applications". We are aware of its content and have made substantial intellectual contributions to its development. We understand that the submission of this manuscript is being made to the Stem Cell Review and Reports Journal and that it is subject to the journal's peer-review process and editorial policies. We acknowledge that we have reviewed and complied with the journal's author guidelines and ethical standards for manuscript submission, including the guidelines on authorship, originality, and conflicts of interest. We understand that the manuscript may be reviewed by independent experts and that revisions may be required before publication. We agree that the corresponding author, Ayberk Akat Ph.D., is authorized to act on our behalf regarding all matters related to the submission, review, and publication of this manuscript, including correspondence with the journal's editorial team. We affirm that the content of this manuscript is original, has not been previously published elsewhere, and is not under consideration for publication in any other journal. We understand that if the manuscript is accepted for publication, it will be made available to the public through the Stem Cell Review and Reports Journal and other platforms. We grant the Stem Cell Review and Reports Journal the right to use and distribute the manuscript in accordance with its publication policies, including online and in print. We confirm that all co-authors have been informed of and consent to the submission of this manuscript to the Stem Cell Review and Reports Journal. We acknowledge that any changes to authorship, conflicts of interest, or other relevant information will be promptly communicated to the journal's editorial team. We hereby provide our consent to participate in the submission of this manuscript and confirm that we have reviewed and agree to the terms outlined above.
Consent for Publication
All authors reviewed the results and approved the final version of the manuscript to be published. All authors hereby give consent for the publication of our personal information in the Stem Cell Review and Reports Journal. This information includes, but is not limited to, our name, age, sex, and any other information that is included in the article. We also understand that we are granting the publisher the exclusive right to publish the article in all languages, in whole or in part. We retain the right to use the article for our own purposes, such as teaching, lecturing, and presenting at conferences.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akat, A., Karaöz, E. Cell Therapy Strategies on Duchenne Muscular Dystrophy: A Systematic Review of Clinical Applications. Stem Cell Rev and Rep 20, 138–158 (2024). https://doi.org/10.1007/s12015-023-10653-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10653-8