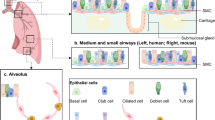
Abstract
Several attempts have been made to reconstruct the whole lung using pluripotent stem cells (PSCs) to treat terminal stage diseases, such as chronic obstructive pulmonary disease [COPD] and idiopathic pulmonary fibrosis [IPF], for which whole-organ transplantation is currently the only treatment option. The development of induced differentiation technologies has made it possible to regenerate lungs from the ‘bottom-up’ via stepwise protocols. Nonetheless, the earliest lung multipotent progenitors, namely lung primordial stem cells, have not been identified to date. Considering the intricate crosstalk network that regulates lung development, stepwise protocols to differentiate PSCs into lung progenitors have raised some key questions: (1) the heterogeneity of these induced progenitors, and (2) obtaining a high-purity population. One important strategy to overcome these hurdles is to identify relevant markers or factors that regulate the complex network in lung morphogenesis according to those erected in vivo and ex vivo experiments. For screening lung primordial stem cells, several markers are ‘on the shelf’, and this review explores the most common or substantiated candidates. We artificially divided these markers into positive selecting and negative limiting proximal or distal markers as well as early progenitor markers that can be used to identify lung primordial stem cell, which represents the earliest progenitor during lung morphogenesis.
Graphical Abstract


Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Kotton, D. N., & Morrisey, E. E. (2014). Lung regeneration: Mechanisms, applications and emerging stem cell populations. Nature Medicine, 20(8), 822–832.
Kumar, V. H., et al. (2005). Growth factors in lung development. Advances in Clinical Chemistry, 40, 261–316.
Schittny, J. C. (2017). Development of the lung. Cell and Tissue Research, 367(3), 427–444.
Morrisey, E. E., & Hogan, B. L. (2010). Preparing for the first breath: Genetic and cellular mechanisms in lung development. Developmental Cell, 18(1), 8–23.
Rankin, S. A., & Zorn, A. M. (2014). Gene regulatory networks governing lung specification. Journal of Cellular Biochemistry, 115(8), 1343–1350.
Bilodeau, C., et al. (2021). TP63 basal cells are indispensable during endoderm differentiation into proximal airway cells on acellular lung scaffolds. NPJ Regenerative Medicine, 6(1), 12.
Vaughan, A. E., et al. (2015). Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature, 517(7536), 621–625.
Shojaie, S., et al. (2015). Acellular lung scaffolds direct differentiation of endoderm to functional airway epithelial cells: Requirement of matrix-bound HS proteoglycans. Stem Cell Reports, 4(3), 419–430.
Inanlou, M. R., Baguma-Nibasheka, M., & Kablar, B. (2005). The role of fetal breathing-like movements in lung organogenesis. Histology and Histopathology, 20(4), 1261–1266.
Li, J., et al. (2018). The Strength of Mechanical Forces Determines the Differentiation of Alveolar Epithelial Cells. Developmental Cell, 44(3), 297-312.e5.
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676.
Hawkins, F., & Kotton, D. N. (2015). Embryonic and Induced Pluripotent Stem Cells for Lung Regeneration. Annals of the American Thoracic Society, 12(Supplement 1), S50–S53.
Ikonomou, L., & Kotton, D. N. (2015). Derivation of Endodermal Progenitors From Pluripotent Stem Cells. Journal of Cellular Physiology, 230(2), 246–258.
Calvert, B.A., Ryan Firth, A.L. (2020). Application of iPSC to Modelling of Respiratory Diseases. Advances in Experimental Medicine and Biology, 1237, 1–16.
Duchesneau, P., Waddell, T. K., Karoubi, G. (2020). Cell-Based Therapeutic Approaches for Cystic Fibrosis. International Journal of Molecular Sciences, 21(15):5219.
Tavakol, D. N., Fleischer, S., & Vunjak-Novakovic, G. (2021). Harnessing organs-on-a-chip to model tissue regeneration. Cell Stem Cell, 28(6), 993–1015.
Parekh, K., et al. (2020). Stem cells and lung regeneration. American journal of physiology. Cell physiology, 319(4), C675–C693.
Basil, M. C., et al. (2020). The Cellular and Physiological Basis for Lung Repair and Regeneration: Past, Present, and Future. Cell Stem Cell, 26(4), 482–502.
Nichane, M., et al. (2017). Isolation and 3D expansion of multipotent Sox9(+) mouse lung progenitors. Nature Methods, 14(12), 1205–1212.
Yang, Y., et al. (2018). Spatial-Temporal Lineage Restrictions of Embryonic p63(+) Progenitors Establish Distinct Stem Cell Pools in Adult Airways. Developmental Cell, 44(6), 752-761.e4.
Hawkins, F., et al. (2017). Prospective isolation of NKX2-1-expressing human lung progenitors derived from pluripotent stem cells. The Journal of Clinical Investigation, 127(6), 2277–2294.
Xi, Y., et al. (2017). Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nature Cell Biology, 19(8), 904–914.
Kim, C. F., et al. (2005). Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell, 121(6), 823–835.
Liu, Q., et al. (2019). Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nature Genetics, 51(4), 728–738.
Salwig, I., et al. (2019). Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo. Embo Journal, 38(12):e102099.
Havrilak, J. A., Melton, K. R., & Shannon, J. M. (2017). Endothelial cells are not required for specification of respiratory progenitors. Developmental Biology, 427(1), 93–105.
Ray, S., et al. (2016). Rare SOX2(+) Airway Progenitor Cells Generate KRT5(+) Cells that Repopulate Damaged Alveolar Parenchyma following Influenza Virus Infection. Stem Cell Reports, 7(5), 817–825.
Herriges, J. C., et al. (2012). Genome-scale study of transcription factor expression in the branching mouse lung. Developmental Dynamics, 241(9), 1432–1453.
Que, J., et al. (2007). Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development, 134(13), 2521–2531.
Tsao, P. N., et al. (2009). Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development, 136(13), 2297–2307.
Tompkins, D. H., et al. (2011). Sox2 activates cell proliferation and differentiation in the respiratory epithelium. American Journal of Respiratory Cell and Molecular Biology, 45(1), 101–110.
Que, J., et al. (2009). Multiple roles for Sox2 in the developing and adult mouse trachea. Development, 136(11), 1899–1907.
Chen, X., Vega, V. B., & Ng, H. H. (2008). Transcriptional regulatory networks in embryonic stem cells. Cold Spring Harbor Symposia on Quantitative Biology, 73, 203–209.
Bhattacharya, S., et al. (2019). SOX2 Regulates P63 and Stem/Progenitor Cell State in the Corneal Epithelium. Stem Cells, 37(3), 417–429.
Nikolić, M.Z., et al. (2017). Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. Elife, 6:e26575.
Alanis, D. M., et al. (2014). Two nested developmental waves demarcate a compartment boundary in the mouse lung. Nature Communications, 5, 3923.
Han, L., et al. (2020). Single cell transcriptomics identifies a signaling network coordinating endoderm and mesoderm diversification during foregut organogenesis. Nature Communications, 11(1), 4158.
Yang, A., et al. (1998). p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Molecular Cell, 2(3), 305–316.
Koster, M. I. (2010). p63 in skin development and ectodermal dysplasias. The Journal of Investigative Dermatology, 130(10), 2352–2358.
Thompson, C. A., DeLaForest, A., & Battle, M. A. (2018). Patterning the gastrointestinal epithelium to confer regional-specific functions. Developmental Biology, 435(2), 97–108.
Evans, M. J., et al. (2001). Cellular and molecular characteristics of basal cells in airway epithelium. Experimental Lung Research, 27(5), 401–415.
Arason, A. J., et al. (2014). deltaNp63 has a role in maintaining epithelial integrity in airway epithelium. PLoS ONE, 9(2), e88683.
Daniely, Y., et al. (2004). Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. American Journal of Physiology. Cell Physiology, 287(1), C171–C181.
Rock, J. R., Randell, S. H., & Hogan, B. L. (2010). Airway basal stem cells: A perspective on their roles in epithelial homeostasis and remodeling. Disease Models & Mechanisms, 3(9–10), 545–556.
Rock, J. R., & Hogan, B. L. (2010). Developmental biology. Branching takes nerve. Science, 329(5999), 1610–1611.
Romano, R. A., et al. (2009). An active role of the DeltaN isoform of p63 in regulating basal keratin genes K5 and K14 and directing epidermal cell fate. PLoS ONE, 4(5), e5623.
Hutton, E., et al. (1998). Functional differences between keratins of stratified and simple epithelia. Journal of Cell Biology, 143(2), 487–499.
Zuo, W., et al. (2015). p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature, 517(7536), 616–620.
Wang, X., et al. (2021). Intrapulmonary distal airway stem cell transplantation repairs lung injury in chronic obstructive pulmonary disease. Cell Proliferation, 54(6), e13046.
Cole, B. B., et al. (2010). Tracheal Basal cells: A facultative progenitor cell pool. American Journal of Pathology, 177(1), 362–376.
Yang, J., et al. (2016). The development and plasticity of alveolar type 1 cells. Development, 143(1), 54–65.
Ghosh, M., et al. (2011). Context-dependent differentiation of multipotential keratin 14-expressing tracheal basal cells. American Journal of Respiratory Cell and Molecular Biology, 45(2), 403–410.
Smirnova, N. F., et al. (2016). Detection and quantification of epithelial progenitor cell populations in human healthy and IPF lungs. Respiratory Research, 17(1), 83.
Ryerse, J. S., et al. (2001). Immunolocalization of CC10 in Clara cells in mouse and human lung. Histochemistry and Cell Biology, 115(4), 325–332.
Liu, K., et al. (2020). Triple-cell lineage tracing by a dual reporter on a single allele. Journal of Biological Chemistry, 295(3), 690–700.
Zheng, D., et al. (2013). A cellular pathway involved in Clara cell to alveolar type II cell differentiation after severe lung injury. PLoS ONE, 8(8), e71028.
Reynolds, S. D., et al. (2000). Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. American Journal of Pathology, 156(1), 269–278.
Rawlins, E. L., et al. (2009). The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell, 4(6), 525–534.
Zheng, D., Yin, L., & Chen, J. (2014). Evidence for Scgb1a1(+) cells in the generation of p63(+) cells in the damaged lung parenchyma. American Journal of Respiratory Cell and Molecular Biology, 50(3), 595–604.
Serls, A. E., et al. (2005). Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development, 132(1), 35–47.
Xu, P., et al. (1998). Morphometric analysis of the immunohistochemical expression of Clara cell 10-kDa protein and surfactant apoproteins A and B in the developing bronchi and bronchioles of human fetuses and neonates. Virchows Archiv, 432(1), 17–25.
Tompkins, D. H., et al. (2009). Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells. PLoS ONE, 4(12), e8248.
Elluru, R. G., & Whitsett, J. A. (2004). Potential role of Sox9 in patterning tracheal cartilage ring formation in an embryonic mouse model. Archives of Otolaryngology - Head and Neck Surgery, 130(6), 732–736.
Wright, E., et al. (1995). The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nature Genetics, 9(1), 15–20.
Rockich, B. E., et al. (2013). Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proceedings of the National Academy of Sciences of the United States of America, 110(47), E4456–E4464.
Bi, W., et al. (2001). Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proceedings of the National Academy of Sciences of the United States of America, 98(12), 6698–6703.
Perl, A. K., et al. (2005). Normal lung development and function after Sox9 inactivation in the respiratory epithelium. Genesis, 41(1), 23–32.
Turcatel, G., et al. (2013). Lung mesenchymal expression of Sox9 plays a critical role in tracheal development. BMC Biology, 11, 117.
Li, L., et al. (2021). SOX9 inactivation affects the proliferation and differentiation of human lung organoids. Stem Cell Research & Therapy, 12(1), 343.
Ma, Q., et al. (2018). Regeneration of functional alveoli by adult human SOX9(+) airway basal cell transplantation. Protein & Cell, 9(3), 267–282.
Kathiriya, J. J., et al. (2020). Distinct Airway Epithelial Stem Cells Hide among Club Cells but Mobilize to Promote Alveolar Regeneration. Cell Stem Cell, 26(3), 346-358.e4.
Barkauskas, C. E., et al. (2013). Type 2 alveolar cells are stem cells in adult lung. The Journal of Clinical Investigation, 123(7), 3025–3036.
Wert, S. E., et al. (1993). Transcriptional elements from the human SP-C gene direct expression in the primordial respiratory epithelium of transgenic mice. Developmental Biology, 156(2), 426–443.
Laresgoiti, U., et al. (2016). Lung epithelial tip progenitors integrate glucocorticoid- and STAT3-mediated signals to control progeny fate. Development, 143(20), 3686–3699.
Liebler, J. M., et al. (2016). Combinations of differentiation markers distinguish subpopulations of alveolar epithelial cells in adult lung. American Journal of Physiology. Lung Cellular and Molecular Physiology, 310(2), L114–L120.
Glasser, S. W., et al. (2001). Altered stability of pulmonary surfactant in SP-C-deficient mice. Proceedings of the National Academy of Sciences of the United States of America, 98(11), 6366–6371.
Rock, J. R., et al. (2011). Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proceedings of the National Academy of Sciences of the United States of America, 108(52), E1475–E1483.
Gonzalez, R., et al. (2019). Cell fate analysis in fetal mouse lung reveals distinct pathways for TI and TII cell development. American Journal of Physiology. Lung Cellular and Molecular Physiology, 317(5), L653-l666.
Treutlein, B., et al. (2014). Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature, 509(7500), 371–375.
Jain, R., et al. (2015). Plasticity of Hopx(+) type I alveolar cells to regenerate type II cells in the lung. Nature Communications, 6, 6727.
Yin, Z., et al. (2006). Hop functions downstream of Nkx2.1 and GATA6 to mediate HDAC-dependent negative regulation of pulmonary gene expression. American Journal of Physiology. Lung Cellular and Molecular Physiology, 291(2), L191–9
Ota, C., et al. (2018). Dynamic expression of HOPX in alveolar epithelial cells reflects injury and repair during the progression of pulmonary fibrosis. Science and Reports, 8(1), 12983.
Stahlman, M. T., Gray, M. E., & Whitsett, J. A. (1996). Expression of thyroid transcription factor-1(TTF-1) in fetal and neonatal human lung. Journal of Histochemistry and Cytochemistry, 44(7), 673–678.
Rankin, S. A., et al. (2018). Timing is everything: Reiterative Wnt, BMP and RA signaling regulate developmental competence during endoderm organogenesis. Developmental Biology, 434(1), 121–132.
Minoo, P., et al. (1999). Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(-/-) mouse embryos. Developmental Biology, 209(1), 60–71.
Li, E., et al. (2021). Blastocyst complementation reveals that NKX2-1 establishes the proximal-peripheral boundary of the airway epithelium. Developmental Dynamics, 250(7), 1001–1020.
Kimura, S., et al. (1996). The T/ebp null mouse: Thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes & Development, 10(1), 60–69.
Wert, S. E., et al. (2002). Increased expression of thyroid transcription factor-1 (TTF-1) in respiratory epithelial cells inhibits alveolarization and causes pulmonary inflammation. Developmental Biology, 242(2), 75–87.
Little, D. R., et al. (2019). Transcriptional control of lung alveolar type 1 cell development and maintenance by NK homeobox 2–1. Proceedings of the National Academy of Sciences of the United States of America, 116(41), 20545–20555.
Little, D. R., et al. (2021). Differential chromatin binding of the lung lineage transcription factor NKX2-1 resolves opposing murine alveolar cell fates in vivo. Nature Communications, 12(1), 2509.
Goh, K. J., et al. (2021). An NKX2-1(GFP) and TP63(tdTomato) dual fluorescent reporter for the investigation of human lung basal cell biology. Science and Reports, 11(1), 4712.
Hawkins, F. J., et al. (2021). Derivation of Airway Basal Stem Cells from Human Pluripotent Stem Cells. Cell Stem Cell, 28(1), 79-95.e8.
Bilodeau, M., et al. (2014). Identification of a proximal progenitor population from murine fetal lungs with clonogenic and multilineage differentiation potential. Stem Cell Reports, 3(4), 634–649.
Song, H., et al. (2012). Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America, 109(43), 17531–17536.
Yao, E., et al. (2018). Notch Signaling Controls Transdifferentiation of Pulmonary Neuroendocrine Cells in Response to Lung Injury. Stem Cells, 36(3), 377–391.
Tanaka, Y., et al. (2018). Characterization of distal airway stem-like cells expressing N-terminally truncated p63 and thyroid transcription factor-1 in the human lung. Experimental Cell Research, 372(2), 141–149.
Ang, S. L., et al. (1993). The formation and maintenance of the definitive endoderm lineage in the mouse: Involvement of HNF3/forkhead proteins. Development, 119(4), 1301–1315.
Monaghan, A. P., et al. (1993). Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development, 119(3), 567–578.
Wan, H., et al. (2005). Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. Journal of Biological Chemistry, 280(14), 13809–13816.
Weinstein, D. C., et al. (1994). The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell, 78(4), 575–588.
Kubo, A., et al. (2004). Development of definitive endoderm from embryonic stem cells in culture. Development, 131(7), 1651–1662.
Wong, A. P., et al. (2012). Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nature Biotechnology, 30(9), 876–882.
Mou, H., et al. (2012). Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell, 10(4), 385–397.
Besnard, V., et al. (2004). Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expression Patterns, 5(2), 193–208.
Uetzmann, L., Burtscher, I., & Lickert, H. (2008). A mouse line expressing Foxa2-driven Cre recombinase in node, notochord, floorplate, and endoderm. Genesis, 46(10), 515–522.
Stahlman, M. T., Gray, M. E., & Whitsett, J. A. (1998). Temporal-spatial distribution of hepatocyte nuclear factor-3beta in developing human lung and other foregut derivatives. Journal of Histochemistry and Cytochemistry, 46(8), 955–962.
Zhou, L., et al. (1997). Hepatocyte nuclear factor-3beta limits cellular diversity in the developing respiratory epithelium and alters lung morphogenesis in vivo. Developmental Dynamics, 210(3), 305–314.
Wan, H., et al. (2004). Foxa2 is required for transition to air breathing at birth. Proceedings of the National Academy of Sciences of the United States of America, 101(40), 14449–14454.
Wan, H., et al. (2004). Foxa2 regulates alveolarization and goblet cell hyperplasia. Development, 131(4), 953–964.
Hao, Y., et al. (2013). Pyocyanin-induced mucin production is associated with redox modification of FOXA2. Respiratory Research, 14(1), 82.
Paranjapye, A., et al. (2020). The FOXA1 transcriptional network coordinates key functions of primary human airway epithelial cells. American Journal of Physiology. Lung Cellular and Molecular Physiology, 319(1), L126-l136.
Serra, M., et al. (2017). Pluripotent stem cell differentiation reveals distinct developmental pathways regulating lung- versus thyroid-lineage specification. Development, 144(21), 3879–3893.
Chen, Y. W., et al. (2017). A three-dimensional model of human lung development and disease from pluripotent stem cells. Nature Cell Biology, 19(5), 542–549.
Park, K. S., et al. (2006). Transdifferentiation of ciliated cells during repair of the respiratory epithelium. American Journal of Respiratory Cell and Molecular Biology, 34(2), 151–157.
Acknowledgements
The author, Yijian Lin, would like to thank all facilities managers, especially Meihua WU in the Stem Cell Laboratory, Second Affiliated Hospital of Fujian Medical University, Chain.
Funding
This work was supported by grants from The General Program (Key program, Major Research Plan) of National Natural Science Foundation of China (82070021), Research Project of Jinan Microecological Biomedicine Shandong Laboratory (JNL-2022020B), Quanzhou City Science & Technology Program of China: Quanzhou High-level talents Project (2020C001R) and Quanzhou High-level talent team project (2020CT001).
Author information
Authors and Affiliations
Contributions
Yijian Lin: Conceptualization, Writing (original draft, review, and editing).
Dachun Wang: Writing (review, and editing), Supervision.
Yiming Zeng: Writing (review, and editing), Supervision.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, Y., Wang, D. & Zeng, Y. A Maverick Review of Common Stem/Progenitor Markers in Lung Development. Stem Cell Rev and Rep 18, 2629–2645 (2022). https://doi.org/10.1007/s12015-022-10422-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10422-z