Abstract
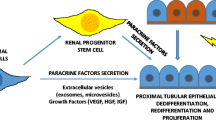
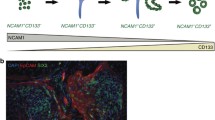
Cell turnover in the healthy adult kidney is very slow but the kidney has a strong capacity for regeneration after acute injury. Although many molecular aspects of this process have been clarified, the source of the newly-formed renal epithelial cells is still being debated. Several studies have shown, moreover, that the repair of injured renal epithelium starts from mature tubular cells, which enter into an activated proliferative state characterized by the reappearance of mesenchymal markers detectable during nephrogenesis, thus pointing to a marked plasticity of renal epithelial cells. The regenerative potential of mature epithelial cells might stem from their almost unique morphogenetic process. Unlike other tubular organs, all epithelial and mesenchymal cells in the kidney derive from the same germ layer, the mesoderm. In a fascinating view of vertebrate embryogenesis, the mesoderm might be seen as a cell layer capable of oscillating between epithelial and mesenchymal states, thus acquiring a remarkable plasticity that lends it an extended potential for innovation and a better control of three-dimensional body organization. The renal papilla contains a population of cells with the characteristic of adult stem cells. Mesenchymal stromal stem cells (MSC) have been found to reside in the connective tissue of most organs, including the kidney. Recent studies indicate that the MSC compartment extends throughout the body postnatally as a result of its perivascular location. Developmental biology suggests that this might be particularly true of the kidney and that the papilla might represent the perivascular renal stem cell niche. The perivascular niche hypothesis fits well with the evolving concept of the stem cell niche as an entity of action. It is its dynamic capability that makes the niche concept so important and essential to the feasibility of regenerative medicine.



Similar content being viewed by others
References
Little, M. H. (2006). Regrow or repair: potential regenerative therapies for the kidney. Journal of the American Society of Nephrology, 17, 2390–401.
Witzgall, R., Brown, D., Schwarz, C., & Bonventre, J. V. (1994). Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. Journal of Clinical Investigation, 93, 2175–2188.
Bonventre, J. V. (2003). Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. Journal of the American Society of Nephrology, suppl 1, S55–S61.
Maeshima, A., Yamashita, S., & Nojima, Y. (2003). Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. Journal of the American Society of Nephrology, 14, 3138–3146.
Oliver, J. A., Maarouf, O., Cheema, F. H., Martens, T. P., & Al-Awqati, Q. (2004). The renal papilla is a niche for adult kidney stem cells. Journal of Clinical Investigation, 114, 795–804.
Dekel, B., Zangi, L., Shezen, E., et al. (2006). Isolation and characterization of non tubular sca-1 + lin- multipotent stem/progenitor cells from adult mouse kidney. Journal of the American Society of Nephrology, 17, 3300–3314.
Gupta, S., Verfaillie, C., Chmielewski, D., et al. (2006). Isolation and characterization of kidney-derived stem cells. Journal of the American Society of Nephrology, 17, 3028–3040.
Poulsom, R., Alison, M. R., Cook, T., et al. (2003). Bone marrow stem cells contribute to healing of the kidney. Journal of the American Society of Nephrology, suppl 1, S48–S54.
McTaggart, S. J., & Atkinson, K. (2007). Mesenchymal stem cells: immunobiology and therapeutic potential in kidney disease. Nephrology, 12, 44–52.
Little, M. H., & Bertram, J. F. (2009). Is there such a thing as a renal stem cell? Journal of the American Society of Nephrology, 20, 2112–2117.
Hishikawa, K., Marumo, T., Miura, S., Nakanishi, A., Matsuzaki, Y., & Shibata, K. (2005). Musculin/MyoR is expressed in kidney side population cells and can regulate their function. The Journal of Cell Biology, 169, 921–928.
Challen, G. A., Bertoncello, I., Deane, J. A., Ricardo, S. D., & Little, M. H. (2006). Kidney side population reveals multilineage potential and renal functional capacity but also cellular heterogeneity. Journal of the American Society of Nephrology, 17, 1896–1912.
Lazzeri, E., Crescioli, C., Ronconi, E., et al. (2007). Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. Journal of the American Society of Nephrology, 18, 3128–3138.
Sagrinati, C., Netti, G. S., Mazzinghi, B., et al. (2006). Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. Journal of the American Society of Nephrology, 17, 2443–2456.
Bruno, S., Bussolati, B., Grange, C., et al. (2009). Isolation and characterization of resident mesenchymal stem cells in human glomeruli. Stem Cells and Development, 18, 867–880.
Bussolati, B., Bruno, S., Grange, C., et al. (2005). Isolation of renal progenitor cells from adult human kidney. The American Journal of Pathology, 166, 545–555.
Kitamura, S., Yamasaki, Y., Kinomura, M., et al. (2005). Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. The FASEB Journal, 2005(19), 1789–1797.
Yamashita, S., Maeshima, A., & Nojima, Y. (2005). Involvement of renal progenitor tubular cells in epithelial-to-mesenchymal transition in fibrotic rat kidneys. Journal of the American Society of Nephrology, 16, 2044–2051.
Markovìc-Lipkovsky, J., Muller, C. A., Klein, G., et al. (2007). Neural cell adhesion molecule expression on renal interstitial cells. Nephrology, Dialysis, Transplantation, 22, 1558–1566.
Lee, P. T., Lin, H. H., Jiang, S. T., et al. (2010). Mouse kidney progenitor cells accelerate renal regeneration and prolong survival after ischemic injury. Stem Cells, 28(3), 573–84.
Ito, M., Liu, Y., Yang, Z., et al. (2005). Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Natural Medicines, 2005(11), 1351–1354.
Crosby, H. A., & Strain, A. J. (2001). Adult liver stem cells: bone marrow, blood, or liver derived? Gut, 48, 153–154.
Vogetseder, A., Karadeniz, A., Kaissling, B., & Le Hir, M. (2005). Tubular cell proliferation in the healthy rat kidney. Histochemistry and Cell Biology, 124, 97–104.
Vogetseder, A., Palan, T., Bacic, D., Kaissling, B., & Le Hir, M. (2007). Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. American Journal of Physiology. Cell Physiology, 292, C807–C813.
Vogetseder, A., Picard, N., Gaspert, A., Walch, M., Kaissling, B., & Le Hir, M. (2008). The proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. American Journal of Physiology. Cell Physiology, 294, C22–C28.
Humphreys, B. D., Valerius, M. T., Kobayashi, A., et al. (2008). Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell, 2, 284–291.
Lin, F., Moran, A., & Igarashi, P. (2005). Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. Journal of Clinical Investigation, 115, 1756–1764.
Anglani, F., Ceol, M., Mezzabotta, F., et al. (2008). The renal stem cell system kidney repair and regeneration. Frontiers in Bioscience, 13, 6395–6405.
Zavadil, J., & Böttinger, E. P. (2005). TGF-beta and epithelial-to-mesenchymal transitions. Oncogene, 24, 5764–5774.
Kalluri, R., & Weinberg, R. A. (2009). The basic of epithelial-mesenchymal transition. Journal of Clinical Investigation, 119, 1420–1428.
Perez-Pomares, J. M., & Munoz-Chapuli, R. (2002). Epithelial-mesenchymal transition: A mesodermal cell strategy for evolutive innovation in metazoans. The Anatomical Record, 268, 343–351.
Acloque, H., & Adams, M. S. (2009). Epithelial-mesenchymal transition: the importance of changing cell state in development and disease. Journal of Clinical Investigation, 119, 1438–1449.
Hay, E. D. (1968). Organization and fine structure of epithelium and mesenchyme in the developing chick embryo. In R. Fleischmajer & R. E. Billingham (Eds.), Epithelial-mesenchymal interactions (pp. 31–55). Baltimore: Williams and Wilkins.
Gilbert, S. F. (1995). Developmental biology (p. 894). Sunderland: Sinauer Associates.
Mani, S. A., Guo, W., Liao, M. J., et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell, 133(4), 704–15.
Swetha, G., Chandra, V., Phadnis, S., & Bhonde, R. (2009). Glomerular parietal epithelial cells of adult murine kidney undergo EMT to generate cells with traits of renal progenitors. J Cell Mol Med. Oct 16 [Epub ahead of print]
Bianchi, G., Muraglia, A., Daga, A., Corte, G., Cancedda, R., & Quarto, R. (2001). Microenvironment and stem properties of bone-marrow derived mesenchymal cells. Wound Repair and Regeneration, 9, 460–466.
Oliver, J. A., Klinakis, A., Cheema, F. K., et al. (2009). Proliferation and migration of label-retaining cells of the kidney papilla. Journal of the American Society of Nephrology, 20(11), 2315–2327.
Scadden, D. T. (2006). The stem-cell niche as an entity of action. Nature, 441, 1075–1079.
da Silva Meirelles, L., Chagastelles, P. C., & Nardi, N. B. (2006). Mesenchymal stem cells in virtually all post-natal organs and tissues. Journal of Cell Science, 119, 2204–2213.
Crisan, M., Yap, S., Casteilla, L. A., et al. (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell, 3, 301–313.
Diaz-Flores, L., Gutièrrez, R., Madrid, J. F., et al. (2009). Pericytes. Morphofunction, interaction and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopatol, 24, 909–969.
Phinney, D. G., & Prockop, D. J. (2007). Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells, 25, 2896–2902.
Johnson, R. C., Leopold, J. A., & Loscalzo, J. (2006). Vascular calcification: pathobiological mechanisms and clinical implications. Circulation Research, 99, 1044–1059.
Covas, D. T., Panepucci, R. A., Fontes, A. M., et al. (2008). Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene—expression profile with CD146+ perivascular cells and fibroblasts. Experimental Hematology, 36, 642–654.
Plotkin, M. D., & Goligorsky, M. S. (2006). Mesenchymal cells from adult kidney support angiogenesis and differentiate into multiple interstitial cell types including erytropoietin-producing fibroblast. American Journal of Physiology-Renal Physiology, 291, F902–F912.
Chen, J., Park, H. C., Addabbo, F., et al. (2008). Kidney-derived mesenchymal stem cells contribute to vasculogenesis, angiogenesis and endothelial repair. Kidney International, 74, 879–889.
Patschan, D., Michurina, T., Shi, H. K., et al. (2007). Normal distribution and medullary-to-cortical shift of Nestin—expressing cells acute renal ischemia. Kidney International, 71, 744–754.
Huang, Y., Johnston, P., Zhang, B., et al. (2009). Kidney-derived stromal cells modulate dendritic and T cell responses. Journal of the American Society of Nephrology, 20, 831–841.
Park, F., Mattson, D. L., Roberts, L. A., & JR, C. A. W. (1997). Evidence for the presence of smooth muscle α-actin within pericytes of the renal medulla. Am J Physiol Regulatory Integrative Comp Physiol, 273, 1742–1748.
Michos, O. (2009). Kidney development: from ureteric bud formation to branching morphogenesis. Current Opin Genet Dev, 19, 484–490.
Guillaume, R., Bressan, M., & Herzlinger, D. (2009). Paraxial mesoderm contributes stromal cells to the developing kidney. Developmental Biology, 329, 169–175.
Anglani, F., Forino, M., Del Prete, D., Tosetto, E., Torregrossa, R., & D’Angelo, A. (2004). In search of adult renal stem cells. Journal of Cellular and Molecular Medicine, 8, 474–487.
Ekblon, P., & Weller, A. (1991). Ontogeny of tubular interstitial cells. Kidney International, 39, 394–400.
Al-Awati, Q., & Oliver, J. A. (2002). Stem cells in the kidney. Kidney International, 61, 387–397.
Dressler, R. D. (2009). Advances in early kidney specification, development and patterning. Development, 136, 3863–3874.
Cullen-McEwen, L. A., Caruana, G., & Bertran, J. F. (2005). The where, what and why of the developing renal stroma. Experimental Nephrology, 99, e1–e8.
Vaughan, M. R., & Quaggin, S. E. (2008). How do mesangial and endothelial cells form glomerular tuft. Journal of the American Society of Nephrology, 19, 24–33.
Tonlorenzi, R., Dellavalle, A., Schnapp, E., Cossu, G., & Sampaolesi, M. (2007). Isolation and characterization of mesoangioblasts from mouse, dog and human tissue. Curr Proc Stem Biol, chapter 2:unit 2B.1.
Brunelli, S., Tagliafico, E., De Angelis, F. G., et al. (2004). Msx2 and necdin combined activities are required for smooth muscle differentiation in mesoangioblast stem cells. Circulation Research, 94, 1571–1578.
Acknowledgements
This study was supported by Grant No. CPDA085494 from the University of Padua.
Conflicts of interest
The authors declare no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anglani, F., Mezzabotta, F., Ceol, M. et al. The Regenerative Potential of the Kidney: What Can We Learn from Developmental Biology?. Stem Cell Rev and Rep 6, 650–657 (2010). https://doi.org/10.1007/s12015-010-9186-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-010-9186-6