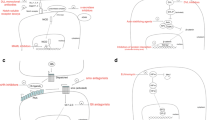
Abstract
As our understanding of embryonic stem cell biology becomes more sophisticated, the similarities between multipotent cancer cells and these totipotent precursors are increasingly striking. Both multipotent cancer cells and embryonic stem cells possess the ability to self-renew, epigenetically alter their neighboring cellular architecture, and populate a tissue mass with a phenotypically heterogeneous composition of cells. While the molecular signature of these cell types continues to be elucidated, new insights are emerging related to the convergence of embryonic and tumorigenic signaling pathways. Understanding the molecular underpinnings of these two stem cell phenotypes may lead to new therapeutic targets for the elusive cancer cell. While still in its infancy, the potential of adapting embryonic stem cells, and more specifically the factors they produce, is enormous for clinical application. Here we outline evidence that demonstrates the inductive influence of embryonic stem cells and their microenvironment to reprogram cancer cells to exhibit a more benign phenotype, with profound implications for differentiation therapy.








Similar content being viewed by others
References
Berlin, A. H. (1858). Die Cellularpathologie in ihrer Begründung auf physiologische und pathologische Gewebelehre. Dritte, neu bearbeitete under vermehrte Auflage, Berlin, 1861. 4th edition, 1871.
Reya, T., Morrison, S. J., Clarke, M. F., & Weissman, I. L. (2001). Stem cells, cancer, and cancer stem cells. Nature, 414(6859), 105–111.
Petersen, B. E., Bowen, W. C., Patrene, K. D., Mars, W. M., Sullivan, A. K., Murase, N., et al. (1999). Bone marrow as a potential source of hepatic oval cells. Science, 284(5417), 1168–1170.
Brazelton, T. R., Rossi, F. M., Keshet, G. I., & Blau, H. M. (2000). From marrow to brain: Adult bone marrow-derived cells give rise to neuronal phenotypes in adult mice. Science, 290(5497), 1775–1779.
Mezey, E., Chandross, K. J., Harta, G., Maki, R. A., & McKercher, S. R. (2000). Turning blood into brain: Cells bearing neuronal antigens generated in vivo from bone marrow. Science, 290(5497), 1779–1782.
Lagasse, E., Connors, H., Al-Dhalimy, M., Reitsma M., Dohse, M., Osborne, L., et al. (2000). Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nature Medicine, 6(11), 1229–1234.
Krause, D. S., Theise, N. D., Collector, M. I., Henegariu, O., Hwang, S., Gardner, R., et al. (2001). Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell, 105(3), 369–377.
Sell, S. (2006). Potential gene therapy strategies for cancer stem cells. Current Gene Therapy, 6(5), 579–591.
Lapidot, T., Sirard, C., Vormoor, J., Murdoch, B., Hoang, T., Caceres-Cortes, J., et al. (1994). A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature, 367(6464), 645–648.
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J., & Clarke, M. F. (2003). From the cover: Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences, 100(7), 3983–3988.
Singh, S. K., Hawkins, C., Clarke, I. D., Squire, J. A., Bayani, J., Hide, T., et al. (2004). Identification of human brain tumour initiating cells. Nature, 432(7015), 396–401.
Hendrix, M. J. C., Seftor, E. A., Meltzer, P. S., Hess, A. R., Gruman, L. M., Nickoloff, B. J., et al. (2003). The stem cell plasticity of aggressive melanoma tumor cells. In S. Sell (Ed.), Stem cells handbook (pp. 297–306). New Jersey, USA: Humana.
Hendrix, M. J. C., Seftor, E. A., Hess, A. R., & Seftor, R. E. B. (2003). Vasculogenic mimicry and tumour-cell plasticity: Lessons from melanoma. Nature Reviews. Cancer, 3(6), 411–421.
Hendrix, M. J. C., Seftor, E. A., Chu, Y. W., Seftor, R. E. B., Nagle, R. B., McDaniel, K. M., et al. (1992). Coexpression of Vimentin and Keratins by human melanoma tumor cells: Correlation with invasive and metastatic potential. Journal of the National Cancer Institute, 84(3), 165–174.
Fang, D., Nguyen, T. K., Leishear, K., Finko, R., Kulp, A. N., Hotz, S., et al. (2005). A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Research, 65(20), 9328–9337.
Hendrix, M. J. C., Seftor, R. E. B., Seftor, E. A., Gruman, L. M., Lee, L. M., Nickoloff, B. J., et al. (2002). Transendothelial function of human metastatic melanoma cells: Role of the microenvironment in cell-fate determination. Cancer Research, 62(3), 665–668.
Hendrix, M. J. C., Seftor, E. A., Meltzer, P. S., Gardner, L. M. G., Hess, A. R., Kirschmann, D. A., et al. (2001). Expression and functional significance of VE-cadherin in aggressive human melanoma cells: Role in vasculogenic mimicry. Proceedings of the National Academy of Sciences, 98(14), 8018–8023.
Maniotis, A. J., Folberg, R., Hess, A. R., Seftor, E. A., Gardner, L. M. G., Pe'er, J., et al. (1999). Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. American Journal of Pathology, 155(3), 739–752.
Velazquez, O. C., & Herlyn, M. (2003). The vascular phenotype of melanoma metastasis. Clinical & Experimental Metastasis, 20, 229–235.
Bittner, M., Meltzer, P., Chen, Y., Jiang, Y., Seftor, E., Hendrix, M., et al. (2000). Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature, 406(6795), 536–540.
Weeraratna, A. T., Jiang, Y., Hostetter, G., Rosenblatt, K., Duray, P., Bittner, M., et al. (2002). Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell, 1(3), 279–288.
Balint, K., Xiao, M., Pinnix, C. C., Soma, A., Veres, I., Juhasz, I., et al. (2005). Activation of Notch1 signaling is required for ß-catenin-mediated human primary melanoma progression. Journal of Clinical Investigation, 115(11), 3166–3176.
Vallier, L., Reynolds, D., & Pedersen, R. A. (2004). Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Developmental Biology, 275(2), 403–421.
Whitman, M. (2001). Nodal signaling in early vertebrate embryos: Themes and variations. Developmental Cell, 1(5), 605–617.
Postovit, L. M., Seftor, E. A., Seftor, R. E. B., & Hendrix, M. J. C. (2007). Targeting Nodal in malignant melanoma cells. Expert Opinion on Therapeutic Targets, 11(4), 497–505.
Lotem, J., & Sachs, L. (2006). Epigenetics and the plasticity of differentiation in normal and cancer stem cell. Oncogene, 25(59), 7663–7672.
Postovit, L. M., Seftor, E. A., Seftor, R. E. B., Hendrix, M. J. C. (2006). A 3-D model to study the epigenetic effects induced by the microenvironment of human embryonic stem cells. Stem Cells, 24(3), 501–505.
Kulesa, P. M., Kasemeier-Kulesa, J. C., Teddy, J. M., Margaryan, N. V., Seftor, E. A., Seftor, R. E. B., et al. (2006). Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proceedings of the National Academy of Sciences, 103(10), 3752–3757.
Pierce, G. B., Pantazis, C. G., Caldwell, J. E., & Wells, R. S. (1982). Specificity of the control of tumor formation by the blastocyst. Cancer Research, 42(3), 1082–1087.
Gerschenson, M., Graves, K., Carson, S. D., Wells, R. S., & Pierce, G. B. (1986). Regulation of melanoma by the embryonic skin. Proceedings of the National Academy of Sciences, 83(19), 7307–7310.
Mintz, B., & Illmensee, K. (1975). Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proceedings of the National Academy of Sciences, 72(9), 3585–3589.
Seftor, E. A., Brown, K. M., Chin, L., Kirschmann, D. A., Wheaton, W. W., Protopopov, A., et al. (2005). Epigenetic transdifferentiation of normal melanocytes by a metastatic melanoma microenvironment. Cancer Research, 65(22), 10164–10169.
Lee, L. M., Seftor, E. A., Bonde, G., Cornell, R. A., & Hendrix, M. J. C. (2005). The fate of human malignant melanoma cells transplanted into zebrafish embryos: Assessment of migration and cell division in the absence of tumor formation. Developmental Dynamics, 233(4), 1560–1570.
Haldi, M., Ton, C., Seng, W. L., & McGrath, P. (2006). Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis, 9(3), 139–151.
Hendrix, M. J. C., Seftor, E. A., Seftor, R. E. B., Kasemeier-Kulesa, J., Kulesa, P. M., & Postovit, L. M. (2007). Reprogramming metastatic tumor cells with embryonic microenvironments. Nature Reviews Cancer, 7(4), 246–255.
Mesnard, D., Guzman-Ayala, M., & Constam, D. B. (2006). Nodal specifies embryonic visceral endoderm and sustains pluripotent cells in the epiblast bgefore overt axial patterning. Development, 133(13), 2497–2505.
Nawshad, A., Lagamba, D., Polad, A., & Hay, E. D. (2005). Transforming growth factor-ß signaling during epithelial-mesenchymal transformation: Implications for embryogenesis and tumor metastasis. Cells Tissues Organs, 179(1–2), 11–23.
Schier, A. F. (2003). Nodal signaling in vertebrate development. Annual Review of Cell and Developmental Biology, 19, 589–621.
Yeo, C., & Whitman, M. (2001). Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Molecular Cell, 7(5), 949–957.
Bianco, C., Adkins, H. B., Wechselberger, C., Seno, M., Normanno, N., De Luca, A., et al. (2002). Cripto-1 activates nodal- and ALK4-dependent and -independent signaling pathways in mammary epithelial cells. Molecular and Cellular Biology, 22(8), 2586–2597.
Herman, J. G., & Baylin, S. B. (2003). Mechanisms of disease: Gene silencing in cancer in association with promoter hyper methylation. New England Journal of Medicine, 349(21), 2042–2054.
Bibikova, M., Chudin, E., Wu, B., Zhou, L., Garcia, E. W., Liu, Y., et al. (2006). Human embryonic stem cells have a unique epigenetic signature. Genome Research, 16(9), 1075–1083.
Postovit, L.-M., Costa, F. F., Bischof, J. M., Seftor, E. A., Wen, B., Seftor, R. E. B. et al. (2007). The Commonality of plasticity underlying multipotent tumor cells and embryonic stem cells. Journal of Cellular Biochemistry, [Epub. Dec. 2006; doi:10.1002/jcb.21227], (in press).
Topczewska, J. M., Postovit, L. M., Margaryan, N. V., Sam, A., Hess, A. R., Wheaton, W. W., et al. (2006). Embryonic and tumorigenic pathways converge via Nodal signaling: Role in melanoma aggressiveness. Nature Medicine, 12(8), 925–932.
Javelaud, D., Delmas, V., Moller, M., Sextius, P., Andre, J., Menashi, S., et al. (2005). Stable overexpression of Smad7 in human melanoma cells inhibits their tumorigenicity in vitro and in vivo. Oncogene, 24(51), 7624–7629.
De Luca, A., Arra, C., D’Antonio, A., Cassamassimi, A., Losito, S., Ferraro, P., et al. (2000). Simultaneous blockade of the different EGF-like growth factors results in efficient growth inhibition of human colon carcinoma xenografts. Oncogene, 19(51), 5863–5871.
Adkins, H. B., Bianco, C., Schiffer, S. G., Rayhorn, P., Zafari, M., Cheung, A. E., et al. (2003). Antibody blockade of the Cripto CFC domain suppresses tumor cell growth in vivo. Journal of Clinical Investigation, 112(4), 575–587.
Xing, P. X., Hu, X. F., Pietersz, G. A., Hosick, H. L., & McKenzie, I. F. (2004). Cripto-1: A novel target for cancer therapy by monoclonal antibodies. Cancer Research, 64(11), 4018–4023.
Halder, S. K., Beauchamp, R. D., & Datta, P. K. (2005). A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia, 7(5), 509–521.
Hjelmeland, M. D., Hjelmeland, A. B., Sathornsumetee, S., Reese, E. D., Herbstreith, M. H., Laping, N. J., et al. (2004). SB-431542, a small molecule transforming growth factor-ß-receptor antagonist, inhibits human glioma cell line proliferation and motility. Molecular Cancer Therapeutics, 3(6), 737–745.
Seftor, R. E. B., Seftor, E. A., Koshikawa, N., Meltzer, P. S., Gardner, L. M. G., Bilban, M., et al. (2001). Cooperative interactions of laminin 5{gamma}2 chain, matrix metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Research, 61(17), 6322–6327.
Postovit, L. M., Seftor, E. A., Seftor, R. E. B., & Hendrix, M. J. C. (2006). Influence of the microenvironment on melanoma cell fate determination and phenotype. Cancer Research, 66(16), 7833–7836.
Seftor, R. E. B., Seftor, E. A., Kirschmann, D. A., & Hendrix, M. J. C. (2002). Targeting the tumor microenvironment with chemically modified tetracyclines: Inhibition of laminin 5{gamma}2 chain promigratory fragments and vasculogenic mimicry. Molecular Cancer Therapeutics, 1(13), 1173–1179.
Golan-Mashiach, M., Dazard, J. E., Gerecht-Nir, S., Amariglio, N., Fisher, T., Jacob-Hirsch, J., et al. (2005). Design principle of gene expression used by human stem cells: Implication for pluripotency. FASEB Journal, 19(1), 147–149.
Tabibzadeh, S., & Hemmati-Brivanlou, A. (2006). Lefty at the crossroads of “stemness” and differentiative events. Stem Cells, 24(9), 1998–2006.
Sato, N., Sanjuan, I. M., Heke, M., Uchida, M., Naef, F., & Brivanlou, A. H. (2003). Molecular signature of human embryonic stem cells and its comparison with the mouse. Developmental Biology, 260(2), 404–413.
Bianco, C., Strizzi, L., Mancino, M., Rehman, A., Hamada, S., Watanabe, K., et al. (2006). Identification of Cripto-1 as a novel serologic marker for breast and colon cancer. Clinical Cancer Research, 12(17), 5158–5164.
Acknowledgements
The authors gratefully acknowledge the scientific interactions with Drs. Paul Kulesa and Jennifer Kasemeier-Kulesa at the Stowers Institute for Medical Research, Drs. Jolanta Topczewska and Jacek Topczewski at the Children’s Memorial Research Center, and Dr. Brian Nickoloff for his expertise in dermatopathology. This work is supported by NIH grant CA59702, an Illinois Regenerative Medicine Institute grant, a Charlotte Geyer Foundation grant (MJCH), and a Canadian Institute for Health Research postdoctoral fellowship (L-MP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abbott, D.E., Postovit, LM., Seftor, E.A. et al. Exploiting the Convergence of Embryonic and Tumorigenic Signaling Pathways to Develop New Therapeutic Targets. Stem Cell Rev 3, 68–78 (2007). https://doi.org/10.1007/s12015-007-0010-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-007-0010-x