Abstract
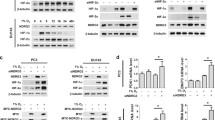
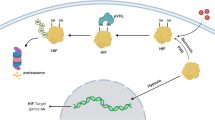
The human kallikrein-related peptidase (KLK) family which consists of 15 members is associated with prostate cancer and other cancers. It has been reported that overexpression of KLK4 in prostate cancer correlates with bone metastasis or advanced stage. Hypoxia occurs in the early stages of prostate cancer due to the accumulation of acidic metabolites or reactive oxygen species (ROS). In our study, KLK4 gene expression in hypoxic conditions in PC-3 and LNCaP cells which are treated with TGF-β was evaluated with mRNA, protein, and promoter activity levels. A chemical hypoxia model was created and confirmed at mRNA and protein level. No statistically significant cytotoxic effect of CoCl2 and TGF-β was observed in PC-3 and LNCaP cells with the MTT test. Four different truncated KLK4 gene promoter constructs were cloned in pmetLuc expression vector and basal activities of all promoter fragments were analyzed. The activities of P1 (−447/ + 657), P2 (−103/ + 657), and P3 (−267/ + 657) promoter fragments increased in hypoxic conditions except P4 (+555/ + 657), which does not contain the SMAD and HRE region. KLK4 mRNA levels in both PC-3 and LNCaP cells increased in the hypoxia and hypoxia/TGF groups compared to the non-treated groups. The stimulating effect of TGF-β is correlated with the increase in SMAD2/3 mRNA levels. KLK4 expression is up-regulated by TGF-β, especially under hypoxic conditions, and its interaction with the SMAD pathway is determined with different inhibitor experiments. HIF-1α and SMAD transcription factors bind to the KLK4 promoter showing the direct interaction of HIF-1α (−80/−52) and SMAD (+163/+194) regions with EMSA.






Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data Availability
The sequence used in KLK4 promoter cloning was obtained from NCBI NG_012154.2 RefSeqGene on Chromosome 10 and the clones were confirmed by sequence analysis. Sequence analysis results can be sent if desired. The KLK4 promoter sequence will also be registered with NCBI.
References
Emami, N., & Diamandis, E. P. (2008). Utility of Kallikrein-Related Peptidases (KLKs) as cancer biomarkers. Clinical Chemistry, 54, 1600–1607. https://doi.org/10.1373/clinchem.2008.105189.
Kontos, C. K., & Scorilas, A. (2012). Kallikrein-related peptidases (KLKs): a gene family of novel cancer biomarkers. Clinical Chemistry and Laboratory Medicine, 50, 1877–1891. https://doi.org/10.1515/cclm-2012-0247.
Kryza, T., Silva, L. M., Bock, N., Fuhrman-Luck, R. A., Stephens, C. R., Gao, J., Samaratunga, H., Lawrence, M. G., Hooper, J. D., Dong, Y., Risbridger, G. P., & Clements, J. A. (2017). Kallikrein-related peptidase 4 induces cancer-associated fibroblast features in prostate-derived stromal cells. Molecular Oncology, 11, 1307–1329. https://doi.org/10.1002/1878-0261.12075.
Fuhrman-Luck, R. A., Stansfield, S. H., Stephens, C. R., Loessner, D., & Clements, J. A. (2016). Prostate Cancer-Associated Kallikrein-Related Peptidase 4 Activates Matrix Metalloproteinase-1 and Thrombospondin-1. Journal of Proteome Research, 15, 2466–2478. https://doi.org/10.1021/acs.jproteome.5b01148.
Okuyan, D., Turkoglu, S. A., & Kockar, F. (2020). Carbonic anhydrase III is a new target of HIF1α in prostate cancer model. Gene, 762, 145034 https://doi.org/10.1016/j.gene.2020.145034.
Selvendiran, K., Bratasz, A., Kuppusamy, M. L., Tazi, M. F., Rivera, B. K., & Kuppusamy, P. (2009). Hypoxia induces chemoresistance in ovarian cancer cells by activation of signal transducer and activator of transcription 3. International Journal of Cancer, 125, 2198–2204. https://doi.org/10.1002/ijc.24601.
Marignol, L., Lawler, M., Coffey, M., & Hollywood, D. (2005). Achieving hypoxiainducible gene expression in tumours. Cancer Biology &Therapy, 4, 365–370. https://doi.org/10.4161/cbt.4.4.1646.
Türkoğlu, S. A., Dayi, G., & Köçkar, F. (2020). Upregulation of PSMD4 gene by hypoxia in prostate cancer cells. Turkish Journal of Biology, 44, 275–283. https://doi.org/10.3906/biy-2002-71.
Turkoglu, S. A., Okuyan, D., & Kockar, F. (2019). TGF-β downregulates CAIII expression via MAPK and PI3K signaling pathways in colon carcinoma and osteosarcoma cells. Archives of Biological Sciences, 71, 393–401. https://doi.org/10.2298/ABS181008020A.
Massague, J. (1998). TGF-β signal transduction. Annual Review of Biochemistry, 67, 753–791. https://doi.org/10.1146/annurev.biochem.67.1.753.
Thompson-Elliott, B., Johnson, R., & Khan, S. A. (2021). Alterations in TGFβ signaling during prostate cancer progression. American Journal of Clinical and Experimental Urology, 9, 318–328.
Massague, J., Seoane, J., & Wotton, D. (2005). Smad transcription factors. Genes & Development, 19, 2783–2810. https://doi.org/10.1101/gad.1350705.
Korkmaz, S. K., Korkmaz, G. C., Pretlow, G. T., & Saatcioglu, F. (2001). Distinctly different gene structure of KLK4/KLK-L1/Prostase/ARM1 compared with other members of the Kallikrein family: intracellular localization, alternative cDNA forms, and regulation by multiple hormones. DNA and Cell Biology, 20, 435–445. https://doi.org/10.1089/104454901750361497.
Suzuki, M., Shin, M., Simmer, J. P., & Bartlett, J. D. (2014). Fluoride affects enamel protein content via TGF-β1-mediated KLK4 inhibition. Journal of Dental Research, 93, 1022–1027. https://doi.org/10.1177/0022034514545629.
Shahinian, H., Loessner, D., Biniossek, M. L., Kizhakkedathu, J. N., Clements, J. A., Magdolen, V., & Schilling, O. (2014). Secretome and degradome profiling shows that Kallikrein-related peptidases 4, 5, 6, and 7 induce TGFβ-1 signaling in ovarian cancer cells. Molecular Oncology, 8, 68–82. https://doi.org/10.1016/j.molonc.2013.09.003.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods, 25, 402–408. https://doi.org/10.1006/meth.2001.1262.
Altuntaş, C., Alper, M., Keleş, Y., Sav, F. N., & Köçkar, F. (2023). Hypoxic regulation of ADAMTS-2 and-3 (a disintegrin and matrix metalloproteinase with thrombospondin motifs 2 and 3) procollagen N proteinases by HIF-1α in endothelial cells. Molecular and Cellular Biochemistry, 478, 1151–1160.
Poyrazlı, F., Türkoğlu, S. A., Babacan, D., & Köçkar, F. (2022). Farklı Hücre Hatlarında KLK4 Gen İfadesinin Belirlenmesi. Karaelmas Fen ve Mühendislik Dergisi, 12, 207–215. https://doi.org/10.7212/karaelmasfen.1080507.
Xi, Z., Klokk, T. I., Korkmaz, K., Kurys, P., Elbi, C., Risberg, B., Danielsen, H., Loda, M., & Saatcioglu, F. (2004). Kallikrein 4 is a Predominantly Nuclear Protein and Is Overexpressed in Prostate Cancer. Cancer Research, 64, 2365–2370. https://doi.org/10.1158/0008-5472.CAN-03-2025.
Dong, Y., Kaushal, A., Bui, L., Chu, S., Fuller, P. J., Nicklin, J., Samaratunga, H., & Clements, J. A. (2001). Human kallikrein 4 (KLK4) is highly expressed in serous ovarian carcinomas. Clinical Cancer Research, 7, 2363–2371.
Lai, J., Myers, S. A., Lawrence, M. G., Odorico, D. M., & Clements, J. A. (2009). Direct progesterone receptor and indirect androgen receptor interactions with the kallikrein-related peptidase 4 gene promoter in breast and prostate cancer. Molecular Cancer Research, 7, 129–141. https://doi.org/10.1158/1541-7786.MCR-08-0218.
Veveris-Lowe, T. L., Lawrence, M. G., Collard, R. L., Bui, L., Herington, A. C., Nicol, D. L., & Clements, J. A. (2005). Kallikrein 4 (hK4) and prostate-specific antigen (PSA) are associated with the loss of E-cadherin and an epithelial-mesenchymal transition (EMT)-like effect in prostate cancer cells. Endocrine-related cancer, 12, 631–643. https://doi.org/10.1677/erc.1.00958.
Mukai, S., Yorit, K., Yamasaki, K., Nagai, T., Kamibeppu, T., Sugie, S., Kida, K., Onizuka, C., Tsukino, H., Kamimura, T., Kamoto, T., & Kataoka, H. (2015). Expression of human kallikrein 1-related peptidase 4 (KLK4) and MET phosphorylation in prostate cancer tissue: immunohistochemical analysis. Human Cell, 28, 133–142.
Obiezu, C. V., Scorilas, A., Katsaros, D., Massobrio, M., Yousef, G. M., Fracchioli, S., Rigault de la Longrais, I. A., Arisio, R., & Diamandis, E. P. (2001). Higher human kallikrein gene 4 (KLK4) expression indicates poor prognosis of ovarian cancer patients. Clinical Cancer Research, 7, 2380–2386.
Avgeris, M., Mavridis, K., & Scorilas, A. (2012). Kallikrein-related peptidases in prostate, breast, and ovarian cancers: from pathobiology to clinical relevance. Biological Chemistry, 393, 301–317. https://doi.org/10.1515/hsz-2011-0260.
Yang, F., Chen, Y., Shen, T., Guo, D., Dakhova, O., Ittmann, M. M., Creighton, C. Z., Zhang, Y., Dang, T. D., & Rowley, D. R. (2014). Stromal TGF-β signaling induces AR activation in prostate cancer. Oncotarget, 5, 10854–10869. https://doi.org/10.18632/oncotarget.2536.
Yousef, G. M., Obiezu, C. V., Luo, L. Y., Black, M. H., & Diamandis, E. P. (1999). Prostase/KLK-L1 is a new member of the human kallikrein gene family, is expressed in prostate and breast tissues, and is hormonally regulated. Cancer Research, 59, 4252–4256.
Nelson, P. S., Gan, L., Ferguson, C., Moss, P., Gelinas, R., Hood, L., & Wang, K. (1999). Molecular cloning and characterization of prostase, an androgen-regulated serine protease with prostate-restricted expression. Proceedings of Nationall Academy of Sciences, 96, 3114–3119. https://doi.org/10.1073/pnas.96.6.3114.
Niu, Y., Yeh, S., Miyamoto, H., Li, G., Altuwaijri, S., Yuan, J., Han, R., Ma, T., Kuo, H. C., & Chang, C. (2008). Tissue prostate-specific antigen facilitates refractory prostate tumor progression via enhancing ARA70-regulated androgen receptor transactivation. Cancer Research, 68, 7110–7119. https://doi.org/10.1158/0008-5472.CAN-07-6507.
Lai, J., An, J., Nelson, C. C., Lehman, M. L., Batra, J., & Clements, J. A. (2014). Analysis of androgen and anti-androgen regulation of KLK-related peptidase 2, 3, and 4 alternative transcripts in prostate cancer. Biological Chemistry, 395, 1127–1132. https://doi.org/10.1515/hsz-2014-0149.
Kryza, T., Silva, M. L., Loessner, D., Heuzé-Vourc’h, N., & Clements, J. A. (2016). The kallikrein-related peptidase family: Dysregulation and functions during cancer progression. Biochimie, 122, 283–299. https://doi.org/10.1016/j.biochi.2015.09.002.
Lawrence, M. G., Lai, J., & Clements, J. A. (2010). Kallikreins on steroids: Structure, function, and hormonal regulation of prostate-specific antigen and the extended kallikrein locus. Endocrine Review, 31, 407–446. https://doi.org/10.1210/er.2009-0034.
Sanchez, W. Y., de Veer, S. J., Swedberg, J. E., Hong, E. J., Reid, J. C., Walsh, T. P., Hooper, J. D., Hammond, G. L., Clements, J. A., & Harris, J. M. (2012). Selective cleavage of human sex hormone-binding globulin by kallikrein-related peptidases and effects on androgen action in LNCaP prostate cancer cells. Endocrinology, 153, 3179–3189. https://doi.org/10.1210/en.2012-1011.
Costanza, B., Umelo, I. A., Bellier, J., Castronovo, V., & Turtoi, A. (2017). Stromal modulators of TGF-beta in cancer. Journal of Clinical Medicine, 6, 7 https://doi.org/10.3390/jcm6010007.
Dallas, S. L., Zhao, S., Cramer, S. D., Chen, Z., Peehl, D. M., & Bonewald, L. F. (2005). Preferential production of latent transforming growth factor beta-2 by primary prostatic epithelial cells and its activation by prostate-specific antigen. Journal of Cellular Physiology, 202, 361–370. https://doi.org/10.1002/jcp.20147.
Park, S. Y., Kim, Y. J., Gao, A. C., Mohler, J. L., Onate, S. A., Hidalgo, A. A., Ip, C., Park, E. M., Yoon, S. Y., & Park, Y. M. (2006). Hypoxia increases androgen receptor activity in prostate cancer cells. Cancer Research, 66, 5121–5129. https://doi.org/10.1158/0008-5472.CAN-05-1341.
Mitani, T., Yamaji, R., Higashimura, Y., Harada, N., Nakano, Y., & Inui, H. (2011). Hypoxia enhances transcriptional activity of androgen receptor through hypoxia-inducible factor-1α in a low androgen environment. The Journal of Steroid Biochemistry and Molecular Biology, 123, 58–64. https://doi.org/10.1016/j.jsbmb.2010.10.009.
Mohamed, O. A., Tesen, H. S., Hany, Sherif, A., Abdelwaha, M. M., & Elnaggar, M. H. (2023). The role of hypoxia on prostate cancer progression and metastasis. Molecular Biology Report, 50, 3873–3884.
Cameron, S., Deblois, G., Hawley, J. R., Qamra, A., Zhou, S., Tonekaboni, S. A. M., Murison, A., Vliet, R. V., Liu, J., Locasale, J. W., & Lupien, M. (2023). Chronic hypoxia favours adoption to a castration-resistant cell state in prostate cancer. Oncogene, 42, 1693–1703.
Ke, Q., & Costa, M. (2006). Hypoxia-inducible factor-1 (HIF-1). Molecular Pharmacology, 70, 1469–1480. https://doi.org/10.1124/mol.106.027029.
Lin, M. F., Meng, T. C., Rao, P. S., Chang, C., Schonthal, A. H., & Lin, F. F. (1998). Expression of human prostatic acid phosphatase correlates with androgen-stimulated cell proliferation in prostate cancer cell lines. Journal of Biological Chemistry, 273, 5939–5947. https://doi.org/10.1074/jbc.273.10.5939.
Chlenski, A., Nakashiro, K., Ketels, K. V., Korovaitseva, G. I., & Oyasu, R. (2001). Androgen receptor expression in androgen-independent pros-tate cancer cell lines. The Prostate, 47, 66–75. https://doi.org/10.1002/pros.1048.
Tilley, W. D., Bentel, J. M., Aspinall, J. O., Hall, R. E., & Horsfall, D. J. (1995). Evidence for a novel mechanism of androgen resistance in thehuman prostate cancer cell line, PC-3. Steroids, 60, 180–186. https://doi.org/10.1016/0039-128X(94)00031-7.
Yuan, S., Trachtenberg, J., Mills, G. B., Brown, T. J., Xu, F., & Keating, A. (1993). Androgen-induced inhibition of cell proliferation in an andro-gen-insensitive prostate cancer cell line (PC-3) transfected with ahuman androgen receptor complementary DNA. Cancer Research, 53, 1304–1311.
Heisler, L. E., Evangelou, A., Lew, A. M., Trachtenberg, J., Elsholtz, H. P., & Brown, T. J. (1997). Androgen-dependent cell cycle arrest and apoptoticdeath in PC-3 prostatic cell cultures expressing a full-lengthhuman androgen receptor. Molecular and Cellular Endocrinology, 126, 59–73. https://doi.org/10.1016/S0303-7207(96)03970-6.
Marcelli, M., Haidacher, S. J., Plymate, S. R., & Birnbaum, R. S. (1995). Alteredgrowth and insulin-like growth factor-binding protein-3 pro-duction in PC3 prostate carcinoma cells stably transfected with aconstitutively active androgen receptor complementary deox-yribonucleic acid. Endocrinology, 136, 1040–1048. https://doi.org/10.1210/en.136.3.1040.
Turkoglu, S. A., & Kockar, F. (2016). SP1 and USF differentially regulate ADAMTS1 gene expression under normoxic and hypoxic conditions in hepatoma cells. Gene, 575, 48–57. https://doi.org/10.1016/j.gene.2015.08.035.
Smale, S. T., & Kadonaga, J. T. (2003). The RNA polymerase II core promoter. Annual Review of Biochemistry, 72, 449–479. https://doi.org/10.1146/annurev.biochem.72.121801.161520.
Thomas, M. C., & Chiang, C. M. (2006). The general transcription machinery and general cofactors. Critical Review in Biochemistry and Molecular Biology, 41, 105–178. https://doi.org/10.1080/10409230600648736.
Acknowledgements
This work was supported by the Turkish Research Council (TUBITAK) project (TBAG 118Z369).
Author information
Authors and Affiliations
Contributions
Sümeyye Aydoğan Türkoğlu designed the research; Fatma Poyrazlı (FP) performed the experiments; Derya Okuyan (DO) performed the IFC experiment; Sümeyye Aydoğan Türkoğlu (SAT) and Feray Köçkar (FK) checked the results; FP, DO and SAT wrote the paper. FK checked the language of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declar no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Poyrazlı, F., Okuyan, D., Köçkar, F. et al. Hypoxic Regulation of the KLK4 Gene in two Different Prostate Cancer Cells Treated with TGF- β. Cell Biochem Biophys 82, 2797–2812 (2024). https://doi.org/10.1007/s12013-024-01396-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-024-01396-5
Key words
Profiles
- Fatma Poyrazlı View author profile