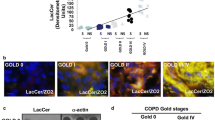
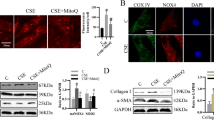
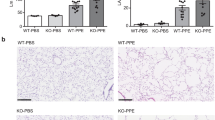
Abstract
Cigarette smoke is the primary cause of Chronic Obstructive Pulmonary Disorder (COPD). Cigarette smoke extract (CSE)-induced oxidative damage of the lungs results in mitochondrial dysfunction and apoptosis of epithelium. Mitochondrial cardiolipin (CL) present in the inner mitochondrial membrane plays an important role in mitochondrial function, wherein its fatty acid composition is regulated by lysocardiolipin acyltransferase (LYCAT). In this study, we investigated the role of LYCAT expression and activity in mitochondrial oxidative stress, mitochondrial dynamics, and lung epithelial cell apoptosis. LYCAT expression was increased in human lung specimens from smokers, and cigarette smoke-exposed-mouse lung tissues. Cigarette smoke extract (CSE) increased LYCAT mRNA levels and protein expression, modulated cardiolipin fatty acid composition, and enhanced mitochondrial fission in the bronchial epithelial cell line, BEAS-2B in vitro. Inhibition of LYCAT activity with a peptide mimetic, attenuated CSE-mediated mitochondrial (mt) reactive oxygen species (ROS), mitochondrial fragmentation, and apoptosis, while MitoTEMPO attenuated CSE-induced MitoROS, mitochondrial fission and apoptosis of BEAS-2B cells. Collectively, these findings suggest that increased LYCAT expression promotes MitoROS, mitochondrial dynamics and apoptosis of lung epithelial cells. Given the key role of LYCAT in mitochondrial cardiolipin remodeling and function, strategies aimed at inhibiting LYCAT activity and ROS may offer an innovative approach to minimize lung inflammation caused by cigarette smoke.









Similar content being viewed by others
Data Availability
All data presented and discussed are contained within the manuscript. All the data and materials are available from V.N.
References
Wohnhaas, C. T., Gindele, J. A., Kiechle, T., Shen, Y., Leparc, G. G., & Stierstorfer, B., et al. (2021). Cigarette Smoke specifically affects small airway epithelial cell populations and triggers the expansion of inflammatory and squamous differentiation associated basal cells. International Journal of Molecular Sciences, 22, 14.
Tanoue, L. T. (2021). Women and lung cancer. Clinics in Chest Medicine, 42(3), 467–82.
Suryadevara, V., Ramchandran, R., Kamp, D. W., & Natarajan, V. (2020). Lipid mediators regulate pulmonary fibrosis: potential mechanisms and signaling pathways. International Journal of Molecular Sciences, 21, 12.
Masunaga, A., Takemura, T., Ichiyasu, H., Migiyama, E., Horio, Y., & Saeki, S., et al. (2021). Pathological and clinical relevance of selective recruitment of Langerhans cells in the respiratory bronchioles of smokers. Respiratory Investigation, 59(4), 513–21.
Chakraborty, R. K., Basit, H., & Sharma, S. (2021). Desquamative Interstitial Pneumonia. Treasure Island (FL): StatPearls.
Shadrach, B. J., Agnihotri, D., Goel, R., & Haran, H. (2021). Pulmonary langerhans cell histiocytosis in a young non-smoking female—too many rituals spoil the lung. Acta Biomedica, 92(S1), e2021138.
Maugeri, G., D’Amico, A. G., Rasa, D. M., La Cognata, V., Saccone, S., & Federico, C., et al. (2017). Nicotine promotes blood retinal barrier damage in a model of human diabetic macular edema. Toxicology In Vitro, 44, 182–9.
Han, X., Cai, C., Huang, J., Li, Q., Huang, L., & Xuan, Q., et al. (2021). The intervention effect of nicotine on cervical fibroblast-myofibroblast differentiation in lipopolysaccharide-induced preterm birth model through activating the TGF-beta1/Smad3 pathway. Biomedicine Pharmacotherapy, 134, 111135.
Lu, X., Zhang, Y., Li, H., Jin, Y., Zhao, L., & Wang, X. (2021). Nicotine prevents in vivo Abeta toxicity in Caenorhabditis elegans via SKN-1. Neuroscience Letter, 761, 136114.
Flenley, D. C., Downing, I., & Greening, A. P. (1986). The pathogenesis of emphysema. Bulletin Europeen de Physiopathologie Respiratoire, 22(1), 245s–52s.
Huang, L. S., Kotha, S. R., Avasarala, S., VanScoyk, M., Winn, R. A., & Pennathur, A., et al. (2020). Lysocardiolipin acyltransferase regulates NSCLC cell proliferation and migration by modulating mitochondrial dynamics. Journal of Biological Chemistry, 295(38), 13393–406.
Aravamudan, B., Thompson, M., Sieck, G. C., Vassallo, R., Pabelick, C. M., & Prakash, Y. S. (2017). Functional effects of cigarette smoke-induced changes in airway smooth muscle mitochondrial morphology. Journal of Cellular Physiology, 232(5), 1053–68.
Zhou, W. C., Qu, J., Xie, S. Y., Sun, Y., & Yao, H. W. (2021). Mitochondrial dysfunction in chronic respiratory diseases: implications for the pathogenesis and potential therapeutics. Oxidative Medicine and Cellular Longevity, 2021, 5188306.
Lin, J., Taggart, M., Borthwick, L., Fisher, A., Brodlie, M., & Sassano, M. F., et al. (2021). Acute cigarette smoke or extract exposure rapidly activates TRPA1-mediated calcium influx in primary human airway smooth muscle cells. Scientific Reports, 11(1), 9643.
Jiang, Z., Knudsen, N. H., Wang, G., Qiu, W., Naing, Z. Z. C., & Bai, Y., et al. (2017). Genetic control of fatty acid beta-oxidation in chronic obstructive pulmonary disease. The American Journal of Respiratory Cell and Molecular Biology, 56(6), 738–48.
Solanki, H. S., Advani, J., Khan, A. A., Radhakrishnan, A., Sahasrabuddhe, N. A., & Pinto, S. M., et al. (2017). Chronic cigarette smoke mediated global changes in lung mucoepidermoid cells: a phosphoproteomic analysis. OMICS., 21(8), 474–87.
Cano, M., Datta, S., Wang, L., Liu, T., Flores-Bellver, M., & Sachdeva, M., et al.(2021). Nrf2 deficiency decreases NADPH from impaired IDH shuttle and pentose phosphate pathway in retinal pigmented epithelial cells to magnify oxidative stress-induced mitochondrial dysfunction. Aging Cell, 20(8), e13444.
Suryadevara, V., Huang, L., Kim, S. J., Cheresh, P., Shaaya, M., & Bandela, M., et al. (2019). Role of phospholipase D in bleomycin-induced mitochondrial reactive oxygen species generation, mitochondrial DNA damage, and pulmonary fibrosis. The American Journal of Physiology Lung Cellular and Molecular Physiology, 317(2), L175–L87.
Brown, Z. J., Fu, Q., Ma, C., Kruhlak, M., Zhang, H., & Luo, J., et al. (2018). Carnitine palmitoyltransferase gene upregulation by linoleic acid induces CD4(+) T cell apoptosis promoting HCC development. Cell Death and Diseases, 9(6), 620.
Anzmann A. F., Sniezek O. L., Pado A., Busa V., Vaz F. M., Kreimer S. D., et al. (2021). Diverse mitochondrial abnormalities in a new cellular model of TAFFAZZIN deficiency are remediated by cardiolipin-interacting small molecules. The Journal of Biological Chemistry, 297(3).
Mejia, E. M., Zegallai, H., Bouchard, E. D., Banerji, V., Ravandi, A., & Hatch, G. M. (2018). Expression of human monolysocardiolipin acyltransferase-1 improves mitochondrial function in Barth syndrome lymphoblasts. The Journal of Biological Chemistry, 293(20), 7564–77.
Garlid, A. O., Schaffer, C. T., Kim, J., Bhatt, H., Guevara-Gonzalez, V., & Ping, P. (2020). TAZ encodes tafazzin, a transacylase essential for cardiolipin formation and central to the etiology of Barth syndrome. Gene, 726, 144148.
Yari, H., Ganjalikhany, M. R., & Sadegh, H. (2015). In silico investigation of new binding pocket for mitogen activated kinase kinase (MEK): Development of new promising inhibitors. Computational Biology and Chemistry, 59 Pt A, 185–98.
Cloonan, S. M., & Choi, A. M. K. (2016). Mitochondria in lung disease. The Journal of Clinical Investigation, 126(3), 809–20.
Huang, L. S., Mathew, B., Li, H., Zhao, Y., Ma, S. F., & Noth, I., et al. (2014). The mitochondrial cardiolipin remodeling enzyme lysocardiolipin acyltransferase is a novel target in pulmonary fibrosis. The American Journal of Respiratory and Critical Care Medicine, 189(11), 1402–15.
Ware, L. B., Landeck, M., Koyama, T., Zhao, Z., Singer, J., & Kern, R., et al. (2014). A randomized trial of the effects of nebulized albuterol on pulmonary edema in brain-dead organ donors. The American Journal of Transplantation, 14(3), 621–8.
Testai, F. D., Xu, H. L., Kilkus, J., Suryadevara, V., Gorshkova, I., & Berdyshev, E., et al. (2015). Changes in the metabolism of sphingolipids after subarachnoid hemorrhage. The Journal of Neuroscience Research, 93(5), 796–805.
Fu, P., Epshtein, Y., Ramchandran, R., Mascarenhas, J. B., Cress, A. E., & Jacobson, J., et al. (2021). Essential role for paxillin tyrosine phosphorylation in LPS-induced mitochondrial fission, ROS generation and lung endothelial barrier loss. Scientific Report, 11(1), 17546.
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry Physiology, 37(8), 911–7.
Schmid, H. H., Schmid, P. C., & Natarajan, V. (1990). N-acylated glycerophospholipids and their derivatives. Progress in Lipid Research, 29(1), 1–43.
Fiske, C. H., & Subbarow, Y. (1925). The colorimetric determination of phosphorus. Journal of Biological Chemistry, 66(2), 375–400.
Suryadevara, V., Fu, P., Ebenezer, D. L., Berdyshev, E., Bronova, I. A., & Huang, L. S., et al. (2018). Sphingolipids in ventilator induced lung injury: role of sphingosine-1-phosphate lyase. International Journal of J Molecular Sciences, 19, 1.
Huang, L. S., Jiang, P., Feghali-Bostwick, C., Reddy, S. P., Garcia, J. G. N., & Natarajan, V. (2017). Lysocardiolipin acyltransferase regulates TGF-beta mediated lung fibroblast differentiation. Free Radical Biology and Medicine, 112, 162–73.
de Groot, P. M., Wu, C. C., Carter, B. W., & Munden, R. F. (2018). The epidemiology of lung cancer. Translational Lung Cancer Research, 7(3), 220–33.
Bandela, M., Letsiou, E., Natarajan, V., Ware, L. B., Garcia, J. G., Singla, S., & Dudek, S. M. (2021). Cortactin ModulatesLung Endothelial Apoptosis Induced by Cigarette Smoke. Cells, 10(11), 2869.
Jezek, J., Cooper, K. F., & Strich, R. (2018). Reactive oxygen species and mitochondrial dynamics: the Yin and Yang of mitochondrial dysfunction and cancer progression. Antioxidants, 7, 1.
Sakhatskyy, P., Gabino Miranda, G. A., Newton, J., Lee, C. G., Choudhary, G., & Vang, A., et al. (2014). Cigarette smoke-induced lung endothelial apoptosis and emphysema are associated with impairment of FAK and eIF2alpha. Microvascular Research, 94, 80–9.
Jassem, E., Szymanowska, A., Sieminska, A., & Jassem, J. (2009). Smoking and lung cancer. Pneumonologia Alergologia Polska, 77(5), 469–73.
Vij, N., Chandramani-Shivalingappa, P., Van Westphal, C., Hole, R., & Bodas, M. (2018). Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Americal Journal of Physiology Cell Physiology, 314(1), C73–C87.
Sundar, I. K., Mullapudi, N., Yao, H., Spivack, S. D., & Rahman, I. (2011). Lung cancer and its association with chronic obstructive pulmonary disease: update on nexus of epigenetics. Current Opinion in Pulmonary Medicine 17(4), 279–85.
Maremanda, K. P., Sundar, I. K., & Rahman, I. (2019). Protective role of mesenchymal stem cells and mesenchymal stem cell-derived exosomes in cigarette smoke-induced mitochondrial dysfunction in mice. Toxicology and Applied Pharmacology, 385, 114788.
Araya, J., Tsubouchi, K., Sato, N., Ito, S., Minagawa, S., & Hara, H., et al. (2019). PRKN-regulated mitophagy and cellular senescence during COPD pathogenesis. Autophagy, 15(3), 510–26.
Aghapour, M., Remels, A. H. V., Pouwels, S. D., Bruder, D., Hiemstra, P. S., & Cloonan, S. M., et al. (2020). Mitochondria: at the crossroads of regulating lung epithelial cell function in chronic obstructive pulmonary disease. The American Journal of Physiology Lung Cellular and Molecular Physiology, 318(1), L149–L64.
Chen, H., Li, Z., Dong, L., Wu, Y., Shen, H., & Chen, Z. (2019). Lipid metabolism in chronic obstructive pulmonary disease. The International Journal of Chronic Obstructive Pulmonary Disease, 14, 1009–18.
Koike, K., Berdyshev, E. V., Bowler, R. P., Scruggs, A. K., Cao, D., & Schweitzer, K. S., et al. (2018). Bioactive sphingolipids in the pathogenesis of chronic obstructive pulmonary disease. The Annals of the American Thoracic Society, 15(Suppl 4), S249–S52.
Petrache, I., Natarajan, V., Zhen, L., Medler, T. R., Richter, A. T., & Cho, C., et al. (2005). Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nature Medicine, 11(5), 491–8.
Ren, M., Phoon, C. K., & Schlame, M. (2014). Metabolism and function of mitochondrial cardiolipin. Progress in Lipid Research, 55, 1–16.
Birk, A. V., Chao, W. M., Bracken, C., Warren, J. D., & Szeto, H. H. (2014). Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. British Journal of Pharmacology, 171(8), 2017–28.
Li, J., Liu, X., Wang, H., Zhang, W., Chan, D. C., & Shi, Y. (2012). Lysocardiolipin acyltransferase 1 (ALCAT1) controls mitochondrial DNA fidelity and biogenesis through modulation of MFN2 expression. Proceedings of National Academy of Sciences of the United States of America, 109(18), 6975–80.
Sedlak, E., & Musatov, A. (2017). Inner mechanism of protection of mitochondrial electron-transfer proteins against oxidative damage. Focus on hydrogen peroxide decomposition. Biochimie, 142, 152–7.
Brookes, P. S., Yoon, Y., Robotham, J. L., Anders, M. W., & Sheu, S. S. (2004). Calcium, ATP, and ROS: a mitochondrial love-hate triangle. The American Journal of Physiology Cell Physiology, 287(4), C817–33.
Cloonan, S. M., & Choi, A. M. (2016). Mitochondria in lung disease. The Journal of Clinical Investigation, 126(3), 809–20.
Cloonan, S. M., Kim, K., Esteves, P., Trian, T., & Barnes, P. J. (2020). Mitochondrial dysfunction in lung ageing and disease. European Respiratory Review, 29, 157.
Dadsena, S., Zollo, C., & Garcia-Saez, A. J. (2021). Mechanisms of mitochondrial cell death. Biochemical Society Transactions, 49(2), 663–74.
Kagan, V. E., Tyurin, V. A., Jiang, J., Tyurina, Y. Y., Ritov, V. B., & Amoscato, A. A., et al. (2005). Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nature Chemical Biology, 1(4), 223–32.
Ye, C., Shen, Z., & Greenberg, M. L. (2016). Cardiolipin remodeling: a regulatory hub for modulating cardiolipin metabolism and function. Journal of Bioenergetics and Biomembranes, 48(2), 113–23.
Baile, M. G., Whited, K., & Claypool, S. M. (2013). Deacylation on the matrix side of the mitochondrial inner membrane regulates cardiolipin remodeling. Molecular Biology of the Cell, 24(12), 2008–20.
Scorrano, L. (2008). Caspase-8 goes cardiolipin: a new platform to provide mitochondria with microdomains of apoptotic signals? Journal of Cell Biology, 183(4), 579–81.
Duncan, A. L. (2020). Monolysocardiolipin (MLCL) interactions with mitochondrial membrane proteins. Biochemical Society Transactions, 48(3), 993–1004.
Song, C., Zhang, J., Qi, S., Liu, Z., Zhang, X., & Zheng, Y., et al. (2019). Cardiolipin remodeling by ALCAT1 links mitochondrial dysfunction to Parkinson’s diseases. Aging Cell, 18(3), e12941.
Zou, C., Synan, M. J., Li, J., Xiong, S., Manni, M. L., & Liu, Y., et al. (2016). LPS impairs oxygen utilization in epithelia by triggering degradation of the mitochondrial enzyme Alcat1. Journal of Cell Science, 129(1), 51–64.
Acknowledgements
This work made use of the instrumentation provided by the Core Facility of the University of Illinois at Chicago’s Research Resources Center.
Author contributions
Conceptualization—M.B., V.N., and B.K.; methodology—M.B., V.S., P.F.U., V.N., R.R., L.H., and S.S.; formal analysis—M.B., R.R., S.M., P.V.S., and V.N.; investigation—M.B., V.N., B.K.; resources—V.N., B.K., S.P.R., and S.M.D.; writing—M.B., V.N., and R.R.; writing (review and editing)—B.M., R.R., and V.N.; project administration—V.N.; funding acquisition—V.N.
Funding
This work was supported by National Institutes of Health grants HLBI P01HL126609, P01HL060678, R01HL127342 (to V.N.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bandela, M., Suryadevara, V., Fu, P. et al. Role of Lysocardiolipin Acyltransferase in Cigarette Smoke-Induced Lung Epithelial Cell Mitochondrial ROS, Mitochondrial Dynamics, and Apoptosis. Cell Biochem Biophys 80, 203–216 (2022). https://doi.org/10.1007/s12013-021-01043-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-021-01043-3