Abstract
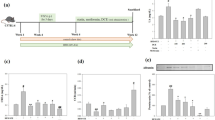
Lasia spinosa (L.) Thwaites is a medicinal plant of enormous traditional use with insufficient scientific evidence. This research screened the antioxidative effect of L. spinosa extracts by measuring the total phenolic content, total flavonoid content, DPPH free radical scavenging activity, ABTS scavenging activity, Iron-chelating activity, and Ferric reducing power followed by an evaluation of in vivo cardioprotective effect in doxorubicin-induced Wistar Albino rats. Phytochemical characterization was made by Gas Chromatography–Mass Spectroscopic analysis. L. spinosa showed an excellent antioxidative effect while methanol leaf extract (LSM) was found to be more potent than ethyl acetate leaf extract (LSE) in scavenging the free radicals. Intraperitoneal injection of doxorubicin caused a significant (P < 0.001) increase in lactate dehydrogenase (LDH), creatine kinase (CK-MB), C-reactive protein (CRP), and Cardiac troponin I. Pretreatment with orally administrated (LSM100 and LSM200 mg/kg b.w.) daily for 10 days showed a decrease in the cardiac markers, lipid profiles, especially triglycerides (TG), total cholesterol (TC), low-density lipoprotein (LDL), and an increase of high-density lipoprotein (HDL) compared to the disease control group. LSM200 was found to significantly (P < 0.05) decrease the levels of CK-MB and LDH. It also restored TC, TG, and LDL levels compared to the doxorubicin-induced cardiac control group. The protective role of LSM was further confirmed by histopathological examination. This study thus demonstrates that L. spinosa methanol extract could be approached as an alternative supplement for cardiotoxicity, especially in the chemical-induced toxicity of cardiac tissues.






Similar content being viewed by others
Data Availability
Data will be available upon request.
Abbreviations
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- ABTS:
-
2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- LDH:
-
Lactate dehydrogenase
- CK-MB:
-
Creatine kinase
- CRP:
-
C-reactive protein
- LSM:
-
L. spinosa Methanol extract
- ESL:
-
L. spinosa Ethyl acetate leaf extract
- TG:
-
Triglyceride
- TC:
-
Total cholesterol
- LDL:
-
Low-density lipoprotein
- HDL:
-
High-density lipoprotein
- DOX:
-
Doxorubicin
- ROS:
-
Reactive oxygen species
- ETC:
-
Electron transport chain
- NADH:
-
Nicotinamide adenine dinucleotide
- eNOS:
-
Endothelial Nitric Oxide Synthase
- NO:
-
Nitric oxide
- GC–MS:
-
Gas chromatography–mass spectrometry
- EI:
-
Electron impact ionization
- TPC:
-
Total phenolic content
- FCR:
-
Folin–Ciocalteu Reagents
- GAE:
-
Gallic acid equivalent
- TFC:
-
Total flavonoid content
- QE:
-
Quercetin equivalent
- IC50 :
-
The half-maximal inhibitory concentration
- OECD:
-
Organization for Environmental Control Development
- LD50 :
-
Lethal Dose 50
- IP:
-
Intraperitoneal
- LSM100:
-
L. spinosa Methanol extract (100 mg/kg /day bw)
- LSM200:
-
L. spinosa Methanol extract (200 mg/kg/day bw)
- LSM50:
-
L. spinosa Methanol extract 50 mg/kg/day bw
- LSE100:
-
L. spinosa Ethyl acetate extract 100 mg/kg/day bw
- LSE50:
-
L. spinosa Ethyl acetate extract 50 mg/kg/day bw
- NBF:
-
10% Neutral-buffered formalin
- H & E:
-
Eosin and hematoxylin
References
Nebigil, C. G., & Désaubry, L. (2018). Updates in anthracycline-mediated cardiotoxicity. Frontiers in Pharmacology, 9, 1262.
Gorini, S., De Angelis, A., Berrino, L., Malara, N., Rosano, G., & Ferraro, E. (2018). Chemotherapeutic drugs and mitochondrial dysfunction: Focus on doxorubicin, trastuzumab, and sunitinib. Oxidative Medicine and Cellular Longevity, 2018, 2018.
Uygur, R., Aktas, C., Tulubas, F., Alpsoy, S., Topcu, B., & Ozen, O. A. (2014). Cardioprotective effects of fish omega-3 fatty acids on doxorubicin-induced cardiotoxicity in rats. Human and Experimental Toxicology, 33, 435–445.
Octavia, Y., Tocchetti, C. G., Gabrielson, K. L., Janssens, S., Crijns, H. J., & Moens, A. L. (2012). Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. Journal of Molecular and Cellular Cardiology, 52, 1213–1225.
Volkova, M., & Russell, R. (2011). Anthracycline cardiotoxicity: Prevalence, pathogenesis, and treatment. Current Cardiology Reviews, 7, 214–220.
Sandamali, J.A., Hewawasam, R.P., Jayatilaka, K.A., & Mudduwa, L.K. (2020). Cardioprotective potential of Murraya koenigii (L.) Spreng. leaf extract against doxorubicin-induced cardiotoxicity in rats. Evid Based Complement Alternat Med.
Sacco, G., Bigioni, M., Evangelista, S., Goso, C., Manzini, S., & Maggi, C. A. (2001). Cardioprotective effects of zofenopril, a new angiotensin-converting enzyme inhibitor, on doxorubicin-induced cardiotoxicity in the rat. European Journal of Pharmacology, 414, 71–78.
Berretta, M., Quagliariello, V., Maurea, N., Di Francia, R., Sharifi, S., Facchini, G., Rinaldi, L., Piezzo, M., Manuela, C., Nunnari, G., & Montopoli, M. (2020). Multiple effects of ascorbic acid against chronic diseases: Updated evidence from preclinical and clinical studies. Antioxidants (Basel), 9, 1182.
Quagliariello, V., Berretta, M., Buccolo, S., Iovine, M., Paccone, A., Cavalcanti, E., Taibi, R., Montopoli, M., Botti, G., & Maurea, N. (2021). Polydatin reduces cardiotoxicity and enhances the anticancer effects of sunitinib by decreasing pro-oxidative stress, pro-inflammatory cytokines, and NLRP3 inflammasome expression. Frontiers in Oncology, 11, 680758.
He, H., Luo, Y., Qiao, Y., Zhang, Z., Yin, D., Yao, J., You, J., & He, M. (2018). Curcumin attenuates doxorubicin-induced cardiotoxicity via suppressing oxidative stress and preventing mitochondrial dysfunction mediated by 14-3-3γ. Food & Function, 9, 4404–4418.
Zhang, Q., & Wu, L. (2022). In Vitro and in vivo cardioprotective effects of curcumin against doxorubicin-induced cardiotoxicity: A systematic review. J Oncology, 2022, 7277562.
Kankanamge, S. U., Amarathunga, A. A. M. D. D. N., Sanjeewani, N. A., & Samanmali, B. L. C. (2017). Phytochemical and ethnopharmacological properties of Lasia spinosa (Kohila): A review. World Journal Pharmaceutical Research, 6, 1–9.
Rahman, A., Siddiqui, S. A., Oke-Altuntas, F., Okay, S., & Demirtas, G. Ü. L. F. (2019). Phenolic profile, essential oil composition and bioactivity of Lasia spinosa (L) thwaites. Brazilian Archives of Biology and Technology. https://doi.org/10.1590/1678-4324-2019170757
Ainsworth, E. A., & Gillespie, K. M. (2007). Estimation of total phenolic content and other oxidation substrates in plant tissues using folin-ciocalteu reagent. Nature Protocols, 2, 875–877.
Chang, C.-C., Yang, M.-H., Wen, H.-M., & Chern, J.-C. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis, 2002, 10.
Brand-Williams, W., Cuvelier, M.-E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology, 28, 25–30.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine, 26, 1231–1237.
Benzie, I. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry, 239, 70–76.
Rahman, M. A., Sultana, R., Bin Emran, T., Islam, M. S., Rahman, M. A., Chakma, J. S., Rashid, H. U., & Hasan, C. M. H. (2013). Effects of organic extracts of six Bangladeshi plants on in vitro thrombolysis and cytotoxicity. BMC Complement and Alternative Medicine, 13, 25.
Zaoui, A., Cherrah, Y., Mahassini, N., Alaoui, K., Amarouch, H., & Hassar, M. (2002). Acute and chronic toxicity of N. sativa fixed oil. Phytomedicine, 9(1), 69–74.
Al-Araby, S. Q., Rahman, M. A., Chowdhury, M. A., Das, R. R., Chowdhury, T. A., Hasan, C. M. M., et al. (2020). Padina tenuis (marine alga) attenuates oxidative stress and streptozotocin-induced type 2 diabetic indices in Wistar albino rats. South African Journal of Botany, 128, 87–100.
Zhao, N., Woodle, M. C., & Mixson, A. J. (2018). Advances in delivery systems for doxorubicin. Journal Nanomed Nanotechnology, 9, 519.
Wallace, K. B., Sardão, V. A., & Oliveira, P. J. (2020). Mitochondrial determinants of doxorubicin-induced cardiomyopathy. Circulation Research, 126, 926–941.
Karimi, A., Majlesi, M., & Rafieian-Kopaei, M. (2015). Herbal versus synthetic drugs; beliefs and facts. Journal Nephropharmacology, 4, 27–30.
Sypniewska, G., & Kruszewski, S. (2021). Cardioprotective effects of nutraceuticals: focus on omega-3 polyunsaturated fatty acids. Nutrients, 13, 3184.
Rashid, M. M., Rahman, M. A., Islam, M. S., Hossen, M. A., Reza, A. S. M. A., Ahmed, A. M. A., Alnajeebi, A. M., Babteen, N. A., Khan, M., Aboelenin, S. M., Soliman, M. M., Habib, A. H., & Alharbi, H. F. (2022). Incredible affinity of Kattosh with PPAR-γ receptors attenuates STZ-induced pancreas and kidney lesions evidenced in chemicobiological interactions. Journal of Cellular and Molecular Medicine, 26, 3343–3363.
Katalinic, V., Milos, M., Kulisic, T., & Jukic, M. (2006). Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chemistry, 94, 550–557.
Ahmed, F., & Urooj, A. (2012). Cardioprotective activity of standardized extract of Ficus racemosa stem bark against doxorubicin-induced toxicity. Pharmaceutical Biology, 50, 468–473.
Kalyanaraman, B. (2020). Teaching the basics of the mechanism of doxorubicin-induced cardiotoxicity: Have we been barking up the wrong tree? Redox Biology, 29, 101394.
Ayza, M. A., Balasubramanian, R., & Berhe, A. H. (2020). Cardioprotective effect of Croton macrostachyus stem bark extract and solvent fractions on cyclophosphamide-induced cardiotoxicity in rats. Evid Based Complement Alternative Medicine, 2020, 2020.
Derbali, A., Mnafgui, K., Affes, M., Derbali, F., Hajji, R., Gharsallah, N., et al. (2015). Cardioprotective effect of linseed oil against isoproterenol-induced myocardial infarction in Wistar rats: A biochemical and electrocardiographic study. Journal of Physiology and Biochemistry, 71, 281–288.
Quagliariello, V., De Laurentiis, M., Cocco, S., Rea, G., Bonelli, A., Caronna, A., Lombari, M. C., Conforti, G., Berretta, M., Botti, G., & Maurea, N. (2020). NLRP3 as putative marker of ipilimumab-induced cardiotoxicity in the presence of hyperglycemia in estrogen-responsive and triple-negative breast cancer cells. International Journal of Molecular Sciences, 21, 7802.
Mehdizadeh, R., Parizadeh, M.-R., Khooei, A.-R., Mehri, S., & Hosseinzadeh, H. (2013). Cardioprotective effect of saffron extract and safranal in isoproterenol-induced myocardial infarction in Wistar rats. Iranian Journal of Basic Medical Sciences, 16, 56.
Priscilla, D. H., & Prince, P. S. M. (2009). Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products, and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chemico-Biological Interactions, 179, 118–124.
Shirole, T. S., Jagtap, A. G., Phadke, A. S., & Velhankar, R. D. (2013). Preventive effect of ethanolic extract of Inula racemosa on electrocardiographic, biochemical, and histopathological alterations in isoproterenol-induced myocardial infarction in rats. International Journal of Research In Phytochemical, 3, 13–22.
Subashini, R., & Rajadurai, M. (2011). Evaluation of cardioprotective efficacy of Nelumbo nucifera leaf extract on isoproterenol-induced myocardial infarction in Wistar rats. International Journal of Pharmaceutical Bioscience, 2, 285–294.
Adegbola, P., Aderibigbe, I., Hammed, W., & Omotayo, T. (2017). Antioxidant and anti-inflammatory medicinal plants have potential role in the treatment of cardiovascular disease: A review. American Journal of Cardiovascular Diseases, 7(2), 19–32.
Ojha, S., Al Taee, H., Goyal, S., Mahajan, U. B., Patil, C. R., Arya, D. S., et al. (2016). Cardioprotective potentials of plant-derived small molecules against doxorubicin associated cardiotoxicity. Oxidative Medicine and Cellular Longevity, 2016, 2016.
Shah, S. M. A., Akram, M., Riaz, M., Munir, N., & Rasool, G. (2019). Cardioprotective potential of plant-derived molecules: A scientific and medicinal approach. Dose-Response, 17, 1559325819852243.
Acknowledgements
The authors wish to thank the Laboratory of Alternative Medicine and Natural Product Research, Department of Biochemistry and Molecular Biology, to support the research’s progress. The authors are also thankful to Dr. Sheikh Bokhtear Uddin, Professor, Department of Botany, for his help to identify the sample, University of Chittagong, Chittagong-4331, Bangladesh,
Funding
NA.
Author information
Authors and Affiliations
Contributions
The research idea was proposed and designed by MAR. The investigation, formal analysis, and data curation were carried out by RA, MKJR, TAS, FYB, and SA. The initial manuscript was authored by MAR, RA, and MKJR, who also contributed to the data analysis. Visualization, validation, and writing—review and editing were done by FYN, MANK, and FS. All authors have gone through the manuscript and agreed to submit it to Cardiovascular Toxicology.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Additional information
Handling Editor: Christian Ellermann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akter, R., Rahman, M.A., Rafi, M.K.J. et al. The Protective Effect of Lasia spinosa (Linn.) Dissipates Chemical-Induced Cardiotoxicity in an Animal Model. Cardiovasc Toxicol 23, 32–45 (2023). https://doi.org/10.1007/s12012-022-09775-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-022-09775-1