Abstract
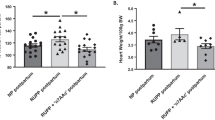
Complete understanding of the etiology underlying endothelial damage in preeclampsia (PE) remains deficient. Recent studies suggest that autoantibodies against angiotensin II AT1 receptors (AT1-AA) may affect vascular endothelial integrity. However, direct evidence demonstrating association between AT1-AA from preeclamptic patients and vascular endothelial injury is lacking. The current study determined the effects of AT1-AA isolated from preeclamptic patients (Pre-IgG) upon the endothelium and attempted to elucidate the underlying mechanisms of injury. Pre-IgG markedly induced dose-dependent vasoconstriction in aortic vascular rings, an effect blocked by AT1 receptor antagonist losartan. Pre-IgG-induced vasoconstriction was increased in the absence of intact endothelium (1.59 ± 0.04 g vs. 1.63 ± 0.08 g, P < 0.05). Additionally, Pre-IgG incubation with human umbilical vein endothelial cells significantly increased lactate dehydrogenase release in a time-dependent manner (0.84 ± 0.07 vs. 3.50 ± 0.09, 24 vs. 72-h exposure group, P < 0.01) and increased caspase-3 and -8 activities (peaking at 48 h), but did not affect caspase-9 activity. Taken together, these results support the contribution of AT1-AA to endothelial cell injury and dysfunction in PE.



Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Hanssens, M., Keirse, M. J., Spitz, B., & Van Assche, F. A. (1991). Measurement of individual plasma angiotensins in normal pregnancy and pregnancy-induced hypertension. Journal of Clinical Endocrinology and Metabolism, 73, 489–494.
Haller, H., Ziegler, E.-M., Homuth, V., Eichhorn, J., Nagy, Z., & Luft, F. C. (1997). Endothelial adhesion molecules and leukocyte integrins in preeclamptic patients. Hypertension, 29, 291–296.
Haller, H., Hempel, A., Homuth, V., Mandelkow, A., Busjahn, A., Maasch, C., et al. (1998). Endothelial cell permeability and protein kinase C in preeclampsia. Lancet, 351, 945–949.
Wallukat, G., Homuth, V., Fischer, T., Lindschau, C., Horstkamp, B., Jüpner, A., et al. (1999). Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. The Journal of Clinical Investigation, 103, 945–952.
Dechend, R., Viedt, C., Müller, D. N., Ugele, B., Brandes, R. P., Wallukat, G., et al. (2003). AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation, 107, 1632–1639.
Thway, T. M., Shlykov, S. G., Day, M. C., Sanborn, B. M., Gilstrap, L. C, 3rd, Xia, Y., et al. (2004). Antibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activation. Circulation, 110, 1612–1619.
Maynard, S. E., Min, J. Y., Merchan, J., Lim, K. H., Li, J., Mondal, S., et al. (2003). Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. The Journal of Clinical Investigation, 111, 649–658.
Zhang, S. L., Du, Y. H., Wang, J., Yang, L. H., Yang, X. L., Zheng, R. H., et al. (2010). Endothelial dysfunction induced by antibodies against angiotensin AT1 receptor in immunized rats. Acta Pharmacologica Sinica, 31, 1381–1388.
Kyle, P. M., Buckley, D., Kissane, J., de Swiet, M., & Redman, C. W. (1995). The angiotensin sensitivity test and low-dose aspirin are ineffective methods to predict and prevent hypertensive disorders in nulliparous pregnancy. American Journal of Obstetrics and Gynecology, 173, 865–872.
Li, H. R. (2001). Effects of long-term immunization with synthesized receptor peptide on in vivo cardiac activity in rats. Journal of Shanxi Medical University, 32, 93–99.
Irani, R. A., & Xia, Y. (2008). The functional role of the renin-angiotensin system in pregnancy and preeclampsia. Placenta, 29(9), 763–771.
Zhou, C. C., Zhang, Y., Irani, R. A., Zhang, H., Mi, T., Popek, E. J., et al. (2008). Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nature Medicine, 14(8), 855–862.
Korzeniewski, C., & Callewaert, D. M. (1983). An enzyme-release assay for natural cytotoxicity. Journal of Immunological Methods, 64, 313–320.
Ma, X. L., Gao, F., Nelson, A. H., Lopez, B. L., Christopher, T. A., Yue, T. L., et al. (2001). Oxidative inactivation of nitric oxide and endothelial dysfunction in stroke-prone spontaneous hypertensive rats. The Journal of Pharmacology and Experimental Therapeutics, 298, 879–885.
Kezić, A., Sparić, R., Stojimirović, B., & Milenković, V. (2007). Multiorgan dysfunction in a gravid woman with placental abruption and disseminated intravascular coagulation. Srpski Arhiv za Celokupno Lekarstvo, 135, 465–467.
Anderson, C. M., & Ren, J. (2002). Leptin, leptin resistance and endothelial dysfunction in pre-eclampsia. Cellular and Molecular Biology (Noisy-le-grand), 48, OL323–OL329.
Weinberger, M. H., Kramer, N. J., Petersen, L. P., Cleary, R. E., & Young, P. C. (1976). Sequential changes in the renin–angiotensin–aldosterone systems and plasma progesterone concentration in normal and abnormal human pregnancy. Perspectives in Nephrology and Hypertension, 5, 263–269.
Brown, M. A., Lindheimer, M. D., de Swiet, M., Van Assche, A., & Moutquin, J. M. (2001). The classification and diagnosis of the hypertensive disorders of pregnancy: Statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertension in Pregnancy, 20, 9–10.
Leung, D. W., Parent, A. S., Cachianes, G., Esch, F., Coulombe, J. N., Nikolics, K., et al. (1992). Cloning, expression during development, and evidence for release of a trophic factor for ciliary ganglion neurons. Neuron, 8, 1045–1053.
Liu, H. R., Tao, L., Gao, E., Lopez, B. L., Christopher, T. A., Willette, R. N., et al. (2004). Anti-apoptotic effects of rosiglitazone in hypercholesterolemic rabbits subjected to myocardial ischemia and reperfusion. Cardiovascular Research, 62, 135–144.
Yang, X., Wang, F., Chang, H., Zhang, S., Yang, L., Wang, X., et al. (2008). Autoantibody against AT1 receptor from preeclamptic patients induces vasoconstriction through angiotensin receptor activation. Hypertension, 26, 1629–1635.
Wang, Z., Jiang, H., Chen, S., Du, F., & Wang, X. (2012). The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell, 148, 228–243.
Xu, Z. X., Ding, T., Haridas, V., Connolly, F., & Gutterman, J. U. (2009). A plant triterpenoid, induces cell apoptosis by recruitment of Fas and downstream signaling molecules into lipid rafts. PLoS One, 4, e8532.
Doonan, F., Donovan, M., & Cotter, T. G. (2003). Caspase-independent photoreceptor apoptosis in mouse models of retinal degeneration. Journal of Neuroscience, 23, 5723–5731.
Rodie, V. A., Freeman, D. J., Sattar, N., & Greer, I. A. (2004). Pre-eclampsia and cardiovascular disease: Metabolic syndrome of pregnancy? Atherosclerosis, 175, 189–202.
Acknowledgments
This research is supported by the Natural Science Foundation of Shanxi Province of China (Grant No. 2013011050-5), the key scientific and technological projects of Shanxi Public Health Department (Grant No. 2011112), the Science and Technology Development Foundation of Taiyuan City (Grant No. 12016915), and the Shanxi Province science research project (Grant No. 20130313019-4).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, X., Wang, F., Lau, W.B. et al. Autoantibodies Isolated from Preeclamptic Patients Induce Endothelial Dysfunction via Interaction with the Angiotensin II AT1 Receptor. Cardiovasc Toxicol 14, 21–29 (2014). https://doi.org/10.1007/s12012-013-9229-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-013-9229-8