Abstract
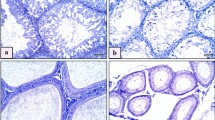
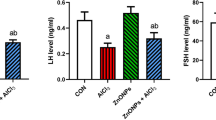
Aluminum chloride is an inorganic polymeric coagulant commonly found in daily life and various materials. Although male reproductive toxicity has been associated with AlCl3 exposure, the underlying mechanism remains unclear. This study aimed to examine the impact of AlCl3 exposure on mitophagy and mitochondrial apoptosis in testicular tissue and mouse spermatocytes. Reactive oxygen species (ROS) and ATP levels were measured in GC-2spd after AlCl3 exposure using a multifunctional enzyme labeler. The changes in mitochondrial membrane potential (MMP) and TUNEL were observed through confocal laser microscopy, and the expression of proteins associated with mitophagy and apoptosis was analyzed using Western blot. Our results demonstrated that AlCl3 exposure disrupted mitophagy and increased apoptosis-related protein expression in testicular tissues. In the in vitro experiments, AlCl3 exposure induced ROS production, suppressed cell viability and ATP production, and caused a decrease in MMP, leading to mitophagy and cell apoptosis in GC-2spd cells. Intervention with N-acetylcysteine (NAC) reduced ROS production and partially restored mitochondrial function, thereby reversing the resulting mitophagy and cell apoptosis. Our findings provide evidence that ROS-mediated mitophagy and cell apoptosis play a crucial role in the toxicity of AlCl3 exposure in GC-2spd. These results contribute to the understanding of male reproductive toxicity caused by AlCl3 exposure and offer a foundation for future research in this area.







Similar content being viewed by others
Data Availability
All data will be provided upon request.
References
Carson SA, Kallen AN (2021) Diagnosis and management of Infertility: A review. JAMA 326:65–76. https://doi.org/10.1001/jama.2021.4788
Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, Vij S, Arafa M, PannerSelvam MK, Shah R (2021) Male infertility. Lancet (London, England) 397:319–333. https://doi.org/10.1016/s0140-6736(20)32667-2
Klotz K, Weistenhöfer W, Neff F, Hartwig A, van Thriel C, Drexler H (2017) The health effects of aluminum exposure. Deutsches Arzteblatt international 114:653–659. https://doi.org/10.3238/arztebl.2017.0653
Nie J (2018) Exposure to aluminum in daily life and Alzheimer’s disease. Adv Exp Med Biol 1091:99–111. https://doi.org/10.1007/978-981-13-1370-7_6
Huston RK, Heisel CF, Vermillion BR, Christensen JM, Minc L (2017) Aluminum content of neonatal parenteral nutrition solutions. Nutr Clin Pract: Off Publ Am Soc Parenteral Enteral Nutr 32:266–270. https://doi.org/10.1177/0884533616668789
Wang YU, Lv H, Lan J, Zhang X, Zhu K, Yang S, Lv S (2022) Detection of sodium formaldehyde sulfoxylate, aluminum, and borate compounds in bread and pasta products consumed by residents in Jilin Province, China. J Food Protect 85:1142–1147. https://doi.org/10.4315/jfp-22-011
Hao W, Hao C, Wu C, Xu Y, Wu S, Lu X, Yang J, Jin C (2021) Aluminum impairs cognitive function by activating DDX3X-NLRP3-mediated pyroptosis signaling pathway. Food Chem Toxicol: Int J Publ Br Ind Biol Res Assoc 157:112591. https://doi.org/10.1016/j.fct.2021.112591
Laabbar W, Abbaoui A, Elgot A, Mokni M, Amri M, Masmoudi-Kouki O, Gamrani H (2021) Aluminum induced oxidative stress, astrogliosis and cell death in rat astrocytes, is prevented by curcumin. J Chem Neuroanat 112:101915. https://doi.org/10.1016/j.jchemneu.2020.101915
Hao W, Hao C, Wu C, Xu Y, Jin C (2022) Aluminum induced intestinal dysfunction via mechanical, immune, chemical and biological barriers. Chemosphere 288:132556. https://doi.org/10.1016/j.chemosphere.2021.132556
Olanrewaju JA, Akinpade TG, Olatunji SY, Owolabi JO, Enya JI, Adelodun ST, Fabiyi SO, Desalu AB (2021) Observable protective activities of quercetin on aluminum chloride-induced testicular toxicity in adult male Wistar rat. J Hum Reprod Sci 14:113–120. https://doi.org/10.4103/jhrs.jhrs_190_20
Huixin P, Guangji W, Yanxin H, Yanfang P, Huixiong Y, Xiong Z, Yu’an X, Wencheng C (2023) Transcriptome-based analysis of the toxic effects of aluminum chloride exposure on spermatocytes. Toxicol In Vitro: Int J Publ Assoc BIBRA 92:105658. https://doi.org/10.1016/j.tiv.2023.105658
Peng H, Huang Y, Wei G, Pang Y, Yuan H, Zou X, Xie Y, Chen W (2023) Testicular toxicity in rats exposed to AlCl(3): a proteomics study. Biol Trace Elem Res. https://doi.org/10.1007/s12011-023-03745-6
Olawuyi TS, Ukwenya VO, Jimoh AGA, Akinola KB (2019) Histomorphometric evaluation of seminiferous tubules and stereological assessment of germ cells in testes following administration of aqueous leaf-extract of Lawsonia inermis on aluminium-induced oxidative stress in adult Wistar rats. JBRA Assist Reprod 23:24–32. https://doi.org/10.5935/1518-0557.20180080
Maghraoui S, Florea A, Ayadi A, Matei H, Tekaya L (2022) Histological and ultrastructural changes observed in testicles, epididymides, seminal vesicles and liver of rat after intraperitoneal administration of aluminum and indium. J Trace Elem Med Biol: Organ Soc Minerals Trace Elem (GMS) 73:126997. https://doi.org/10.1016/j.jtemb.2022.126997
Alizadeh B, Salehzadeh A, Ranji N, Arasteh A (2022) Effects of N-Acetyl cysteine on genes expression of c-myc, and Ask-1, histopathological, oxidative stress, inflammation, and apoptosis in the liver of male rats exposed to cadmium. Biol Trace Elem Res 200:661–668. https://doi.org/10.1007/s12011-021-02670-w
Wang L, Cao J, Chen D, Liu X, Lu H, Liu Z (2009) Role of oxidative stress, apoptosis, and intracellular homeostasis in primary cultures of rat proximal tubular cells exposed to cadmium. Biol Trace Elem Res 127:53–68. https://doi.org/10.1007/s12011-008-8223-7
Jakubczyk K, Dec K, Kałduńska J, Kawczuga D, Kochman J, Janda K (2020) Reactive oxygen species - sources, functions, oxidative damage. Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego 48:124–127
Wang Y, Wang X, Wang L, Cheng G, Zhang M, Xing Y, Zhao X, Liu Y, Liu J (2021) Mitophagy induced by mitochondrial function damage in chicken kidney exposed to Cr(VI). Biol Trace Elem Res 199:703–711. https://doi.org/10.1007/s12011-020-02176-x
Yin J, Ni B, Tian ZQ, Yang F, Liao WG, Gao YQ (2017) Regulatory effects of autophagy on spermatogenesis. Biol Reprod 96:525–530. https://doi.org/10.1095/biolreprod.116.144063
Shiba-Fukushima K, Imai Y, Yoshida S, Ishihama Y, Kanao T, Sato S, Hattori N (2012) PINK1-mediated phosphorylation of the Parkin ubiquitin-like domain primes mitochondrial translocation of Parkin and regulates mitophagy. Sci Rep 2:1002. https://doi.org/10.1038/srep01002
Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, Magrané J, Moore DJ, Dawson VL, Grailhe R, Dawson TM, Li C, Tieu K, Przedborski S (2010) PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA 107:378–383. https://doi.org/10.1073/pnas.0911187107
Ordureau A, Sarraf SA, Duda DM, Heo JM, Jedrychowski MP, Sviderskiy VO, Olszewski JL, Koerber JT, Xie T, Beausoleil SA, Wells JA, Gygi SP, Schulman BA, Harper JW (2014) Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis. Mol Cell 56:360–375. https://doi.org/10.1016/j.molcel.2014.09.007
Anand H, Misro MM, Sharma SB, Prakash S (2015) Protective effects of Eugenia jambolana extract versus N-acetyl cysteine against cisplatin-induced damage in rat testis. Andrologia 47:194–208. https://doi.org/10.1111/and.12247
Zhang Y, Dong F, Wang Z, Xu B, Zhang T, Wang Q, Lin Q (2023) Fluoride exposure provokes mitochondria-mediated apoptosis and increases mitophagy in osteocytes via increasing ROS production. Biol Trace Elem Res 201:3994–4007. https://doi.org/10.1007/s12011-022-03450-w
Lin YC, Lin YC, Tsai ML, Liao WT, Hung CH (2022) TSLP regulates mitochondrial ROS-induced mitophagy via histone modification in human monocytes. Cell Biosci 12:32. https://doi.org/10.1186/s13578-022-00767-w
Bai H, Yang F, Jiang W, Hu A, Chang H, Zhang Y, Jiang L, Lin S, Lu Z, Zhang C, Cao H (2021) Molybdenum and cadmium co-induce mitophagy and mitochondrial dysfunction via ROS-mediated PINK1/Parkin pathway in Hepa1–6 cells. Ecotoxicol Environ Saf 224:112618. https://doi.org/10.1016/j.ecoenv.2021.112618
Wang X, Tian X, Yan H, Zhu T, Ren H, Zhou Y, Zhao D, Xu D, Lian X, Fang L, Yu Y, Liao X, Liu Y, Sun J (2023) Exposure to salinomycin dysregulates interplay between mitophagy and oxidative response to damage the porcine jejunal cells. Sci Total Environ: 166441. https://doi.org/10.1016/j.scitotenv.2023.166441
Xu F, Liu Y, Zhao H, Yu K, Song M, Zhu Y, Li Y (2017) Aluminum chloride caused liver dysfunction and mitochondrial energy metabolism disorder in rat. J Inorg Biochem 174:55–62. https://doi.org/10.1016/j.jinorgbio.2017.04.016
Liu M, Wu X, Cui Y, Liu P, Xiao B, Zhang X, Zhang J, Sun Z, Song M, Shao B, Li Y (2021) Mitophagy and apoptosis mediated by ROS participate in AlCl(3)-induced MC3T3-E1 cell dysfunction. Food Chem Toxicol: Int J Publ Br Ind Biol Res Assoc 155:112388. https://doi.org/10.1016/j.fct.2021.112388
Hao H, Cao L, Jiang C, Che Y, Zhang S, Takahashi S, Wang G, Gonzalez FJ (2017) Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis. Cell Metab 25:856-867.e855. https://doi.org/10.1016/j.cmet.2017.03.007
Huang P, Zhang W, Ji J, Ma J, Cheng H, Qin M, Wei D, Ren L (2023) LncRNA Miat knockdown protects against pirarubicin-induced cardiotoxicity by targeting miRNA-129-1-3p. Environ Toxicol. https://doi.org/10.1002/tox.23910
Huang M, Ivantsova E, Konig I, Patel N, English C, Souders CL 2nd, Martyniuk CJ (2023) Developmental and mitochondrial toxicity assessment of perfluoroheptanoic acid (PFHpA) in zebrafish (Danio rerio). Environ Toxicol Pharmacol 97:104037. https://doi.org/10.1016/j.etap.2022.104037
Xu J, Wang L, Zhang L, Zheng F, Wang F, Leng J, Wang K, Héroux P, Shen HM, Wu Y, Xia D (2021) Mono-2-ethylhexyl phthalate drives progression of PINK1-parkin-mediated mitophagy via increasing mitochondrial ROS to exacerbate cytotoxicity. Redox Biol 38:101776. https://doi.org/10.1016/j.redox.2020.101776
Mandil R, Prakash A, Rahal A, Koli S, Kumar R, Garg SK (2023) Evaluation of oxidative stress-mediated cytotoxicity and genotoxicity of copper and flubendiamide: amelioration by antioxidants in vivo and in vitro. Toxicol Res 12:232–252. https://doi.org/10.1093/toxres/tfad011
Zhou L, He M, Li X, Lin E, Wang Y, Wei H, Wei X (2022) Molecular mechanism of aluminum-induced oxidative damage and apoptosis in rat cardiomyocytes. Biol Trace Elem Res 200:308–317. https://doi.org/10.1007/s12011-021-02646-w
Marrocco I, Altieri F, Peluso I (2017) Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid Med Cell Longev 2017:6501046. https://doi.org/10.1155/2017/6501046
Yang D, Zhu J, Zhou X, Pan D, Nan S, Yin R, Lei Q, Ma N, Zhu H, Chen J, Han L, Ding M, Ding Y (2022) Polystyrene micro- and nano-particle coexposure injures fetal thalamus by inducing ROS-mediated cell apoptosis. Environ Int 166:107362. https://doi.org/10.1016/j.envint.2022.107362
de la Fuente-Herreruela D, Gónzalez-Charro V, Almendro-Vedia VG, Morán M, Martín M, Lillo MP, Natale P, López-Montero I (2017) Rhodamine-based sensor for real-time imaging of mitochondrial ATP in living fibroblasts. Biochim Biophys Acta 1858:999–1006. https://doi.org/10.1016/j.bbabio.2017.09.004
Fang H, Wu Y, Guo J, Rong J, Ma L, Zhao Z, Zuo D, Peng S (2012) T-2 toxin induces apoptosis in differentiated murine embryonic stem cells through reactive oxygen species-mediated mitochondrial pathway. Apoptosis: Int J Program Cell Death 17:895–907. https://doi.org/10.1007/s10495-012-0724-3
Chen J, Yang S, Ma B, Wang J, Chen J (2022) Di-isononyl phthalate induces apoptosis and autophagy of mouse ovarian granulosa cells via oxidative stress. Ecotoxicol Environ Saf 242:113898. https://doi.org/10.1016/j.ecoenv.2022.113898
Huang Y, Mo S, Jin Y, Zheng Z, Wang H, Wu S, Ren Z, Wu J (2022) Ammonia-induced excess ROS causes impairment and apoptosis in porcine IPEC-J2 intestinal epithelial cells. Ecotoxicol Environ Saf 243:114006. https://doi.org/10.1016/j.ecoenv.2022.114006
Li Y, She W, Guo T, Huang T, Liu Y, Liu P, Xu X, Wang X, Wang M, Yu C, Liu Y, Wei Y (2023) The organic arsenical-derived thioredoxin and glutathione system inhibitor ACZ2 induces apoptosis and autophagy in gastric cancer via ROS-dependent ER stress. Biochem Pharmacol 208:115404. https://doi.org/10.1016/j.bcp.2022.115404
Kang L, Liu S, Li J, Tian Y, Xue Y, Liu X (2020) Parkin and Nrf2 prevent oxidative stress-induced apoptosis in intervertebral endplate chondrocytes via inducing mitophagy and anti-oxidant defenses. Life Sci 243:117244. https://doi.org/10.1016/j.lfs.2019.117244
Zhang T, Wu P, Budbazar E, Zhu Q, Sun C, Mo J, Peng J, Gospodarev V, Tang J, Shi H, Zhang JH (2019) Mitophagy reduces oxidative stress via Keap1 (Kelch-Like Epichlorohydrin-Associated Protein 1)/Nrf2 (Nuclear Factor-E2-Related Factor 2)/PHB2 (Prohibitin 2) pathway after subarachnoid hemorrhage in rats. Stroke 50:978–988. https://doi.org/10.1161/strokeaha.118.021590
Yi L, Shang XJ, Lv L, Wang Y, Zhang J, Quan C, Shi Y, Liu Y, Zhang L (2022) Cadmium-induced apoptosis of Leydig cells is mediated by excessive mitochondrial fission and inhibition of mitophagy. Cell Death Dis 13:928. https://doi.org/10.1038/s41419-022-05364-w
Liu T, Hou B, Zhang Y, Wang Z (2022) Determination of biological and molecular attributes related to polystyrene microplastic-induced reproductive toxicity and its reversibility in male mice. Int J Environ Res Public Health 19. https://doi.org/10.3390/ijerph192114093
Cui T, Jiang W, Yang F, Luo J, Hu R, Cao H, Hu G, Zhang C (2021) Molybdenum and cadmium co-induce hypothalamus toxicity in ducks via disturbing Nrf2-mediated defense response and triggering mitophagy. Ecotoxicol Environ Saf 228:113022. https://doi.org/10.1016/j.ecoenv.2021.113022
Zhao Y, Wang J, Zhang J, Sun Z, Niu R, Manthari RK, Ommati MM, Wang S, Wang J (2022) Fluoride exposure induces mitochondrial damage and mitophagy via activation of the IL-17A pathway in hepatocytes. Sci Total Environ 804:150184. https://doi.org/10.1016/j.scitotenv.2021.150184
Cui Y, Li B, Du J, Huo S, Song M, Shao B, Wang B, Li Y (2022) Dibutyl phthalate causes MC3T3-E1 cell damage by increasing ROS to promote the PINK1/Parkin-mediated mitophagy. Environ Toxicol 37:2341–2353. https://doi.org/10.1002/tox.23600
Miao Z, Miao Z, Wang S, Wu H, Xu S (2022) Exposure to imidacloprid induce oxidative stress, mitochondrial dysfunction, inflammation, apoptosis and mitophagy via NF-kappaB/JNK pathway in grass carp hepatocytes. Fish Shellfish Immunol 120:674–685. https://doi.org/10.1016/j.fsi.2021.12.017
Huang Z, Wang S, Yang Y, Lou J, Liu Z, Liu Z, Yong H, Shan S, Song F (2023) Mitochondrial dysfunction promotes the necroptosis of Purkinje cells in the cerebellum of acrylamide-exposed rats. Food Chem Toxicol: Int J Publ Br Ind Biol Res Assoc 171:113522. https://doi.org/10.1016/j.fct.2022.113522
Funding
We thank the Guangxi Natural Science Foundation Project (2020GXNSFAA297257), National Natural Science Foundation of China (81960303), Guangxi University Young and Middle-aged Teachers' Basic Ability Improvement Project (2020KY13017), Guangxi Zhuang Autonomous Region Administration of Traditional Chinese Medicine Self-financing Scientific Research Project (GZZC2020248), and Guangxi Zhuang Autonomous Region Health and Health Commission Self-financing Scientific Research Course (Z20201416).
Author information
Authors and Affiliations
Contributions
Hui-xin Peng, fu Chai and Ke-heng Chen were responsible for experiment operation and paper writing; Yan-xin Huang, Yan-fang Pang and Hui-xiong Yuan were responsible for animal feeding and modeling; Guang-ji Wei was responsible for partial data analysis; Wen-cheng Chen, Chun-fang Wang and Shi-hua Luo were responsible for experiment design, paper writing guidance, overall framework construction and project fund preparation.
Corresponding authors
Ethics declarations
Competing Interests
There is no conflict of interest among the authors of this article, which will not affect the reporting of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hui-xin Peng, Fu Chai and Ke-heng Chen share the frst authorship.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, H.x., Chai, F., Chen, Kh. et al. Reactive Oxygen Species-Mediated Mitophagy and Cell Apoptosis are Involved in the Toxicity of Aluminum Chloride Exposure in GC-2spd. Biol Trace Elem Res 202, 2616–2629 (2024). https://doi.org/10.1007/s12011-023-03848-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03848-0