Abstract
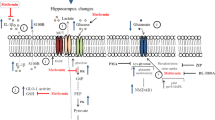
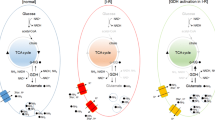
Metformin has been suggested to have protective effects on the central nervous system, but the mechanism is unknown. The similarity between the effects of metformin and the inhibition of glycogen synthase kinase (GSK)-3β suggests that metformin may inhibit GSK-3β. In addition, zinc is an important element that inhibits GSK-3β by phosphorylation. In this study, we investigated whether the effects of metformin on neuroprotection and neuronal survival were mediated by zinc-dependent inhibition of GSK-3β in rats with glutamate-induced neurotoxicity. Forty adult male rats were divided into 5 groups: control, glutamate, metformin + glutamate, zinc deficiency + glutamate, and zinc deficiency + metformin + glutamate. Zinc deficiency was induced with a zinc-poor pellet. Metformin was orally administered for 35 days. D-glutamic acid was intraperitoneally administered on the 35th day. On the 38th day, neurodegeneration was examined histopathologically, and the effects on neuronal protection and survival were evaluated via intracellular S-100β immunohistochemical staining. The findings were examined in relation to nonphosphorylated (active) GSK-3β levels and oxidative stress parameters in brain tissue and blood. Neurodegeneration was increased (p < 0.05) in rats fed a zinc-deficient diet. Active GSK-3β levels were increased in groups with neurodegeneration (p < 0.01). Decreased neurodegeneration, increased neuronal survival (p < 0.01), decreased active GSK-3β (p < 0.01) levels and oxidative stress parameters, and increased antioxidant parameters were observed in groups treated with metformin (p < 0.01). Metformin had fewer protective effects on rats fed a zinc-deficient diet. Metformin may exert neuroprotective effects and increase S-100β-mediated neuronal survival by zinc-dependent inhibition of GSK-3β during glutamate neurotoxicity.









Similar content being viewed by others
Data Availability
The raw data supporting this study will be available by authors to any qualified researcher upon request.
References
Collier CA, Bruce CR, Smith AC, Lopaschuk G, Dyck DJ (2006) Metformin counters the insulin-induced suppression of fatty acid oxidation and stimulation of triacylglycerol storage in rodent skeletal muscle. Am J Physiol Endocrinol Metab 291(1): E182–E189. https://doi.org/10.1152/ajpendo.00272.2005
Russo GL, Russo M, Ungaro P (2013) AMP-activated protein kinase: a target for old drugs against diabetes and cancer. Biochem Pharmacol 86(3):339–350. https://doi.org/10.1016/j.bcp.2013.05.023
Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A (2015) Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis 30(3):747–754. https://doi.org/10.1007/s11011-014-9632-2
Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, Zhang QQ, Gao L, Shi JQ, Zhang YD, Tan L (2014) Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol 171(13):3146–3157. https://doi.org/10.1111/bph.12655
Jin Q, Cheng J, Liu Y, Wu J, Wang X, Wei S, Zhou X, Qin Z, Jia J, Zhen X (2014) Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav Immun 40:131–142. https://doi.org/10.1016/j.bbi.2014.03.003
Sutherland C, Leighton IA, Cohen P (1993) Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J 296(Pt 1):15–19. https://doi.org/10.1042/bj2960015
Grabinski T, Kanaan NM (2016) Novel non-phosphorylated serine 9/21 GSK3β/α antibodies: expanding the tools for studying GSK3 regulation. Front Mol Neurosci 9:123. https://doi.org/10.3389/fnmol.2016.00123
Watcharasit P, Bijur GN, Zmijewski JW, Song L, Zmijewska A, Chen X, Johnson GV, Jope RS (2002) Direct, activating interaction between glycogen synthase kinase-3beta and p53 after DNA damage. Proc Natl Acad Sci USA 99:7951–7955. https://doi.org/10.1073/pnas.122062299
Hanumanthappa P, Densi A, Krishnamurthy RG (2014) Glycogen synthase kinase-β3 in ischemic neuronal death. Curr Neurovasc Res 11(3):271–278. https://doi.org/10.2174/1567202611666140520151002
Chuang DM, Wang Z, Chiu CT (2011) GSK-3 as a target for lithium-induced neuroprotection against excitotoxicity in neuronal cultures and animal models of ischemic stroke. Front Mol Neurosci 4:15. https://doi.org/10.3389/fnmol.2011.00015
Kelly KL, Ruderman NB (1993) Insulin-stimulated phosphatidylinositol 3-kinase. Association with a 185-kDa tyrosine-phosphorylated protein (IRS-1) and localization in a low-density membrane vesicle. J Biol Chem 268(6):4391–4398. https://doi.org/10.1074/jbc.271.19.11222
Frederickson CJ, Koh JY, Bush AI (2005) The neurobiology of zinc in health and disease. Nat Rev Neurosci 6(6):449–462. https://doi.org/10.1038/nrn1671
Jiménez-Jiménez FJ, Molina JA, Aguilar MV, Meseguer I, Mateos-Vega CJ, González-Muñoz MJ, de Bustos F, Martínez-Salio A, Ortí-Pareja M, Zurdo M, Martínez-Para MC (1998) Cerebrospinal fluid levels of transition metals in patients with Parkinson’s disease. J Neural Transm 105(4–5):497–505. https://doi.org/10.1007/s007020050073
Rulon LL, Robertson JD, Lovell MA et al (2000) (2000) Serum zinc levels and Alzheimer’s disease. Biol Trace Elem Res 75:79–85. https://doi.org/10.1385/BTER:75:1-3:79
Ilouz R, Kaidanovich O, Gurwitz D, Eldar-Finkelman H (2002) Inhibition of glycogen synthase kinase-3beta by bivalent zinc ions: insight into the insulin-mimetic action of zinc. Biochem Biophys Res Commun 295(1):102–106. https://doi.org/10.1016/s0006-291x(02)00636-8
Sorci G, Bianchi R, Riuzzi F, Tubaro C, Arcuri C, Giambanco I, Donato R (2010) S100B protein, a damage-associated molecular pattern protein in the brain and heart, and beyond. Cardiovasc Psychiatry Neurol 656481. https://doi.org/10.1155/2010/656481
Garg R, Benedetti LG, Abera MB, Wang H, Abba M, Kazanietz MG (2014) Protein kinase C and cancer: what we know and what we do not. Oncogene 33(45):5225–5237. https://doi.org/10.1038/onc.2013.524
Arcuri C, Bianchi R, Brozzi F, Donato R (2005) S100B increases proliferation in PC12 neuronal cells and reduces their responsiveness to nerve growth factor via Akt activation. J Biol Chem 280(6):4402–4414. https://doi.org/10.1074/jbc.M406440200
Francoz S, Froment P, Bogaerts S, De Clercq S, Maetens M, Doumont G, Bellefroid E, Marine JC (2006) Mdm4 and Mdm2 cooperate to inhibit p53 activity in proliferating and quiescent cells in vivo. Proc Natl Acad Sci USA 103(9):3232–3237. https://doi.org/10.1073/pnas.0508476103
Choi YH, Kim SG, Lee MG (2006) Dose-independent pharmacokinetics of metformin in rats: hepatic and gastrointestinal first-pass effects. J Pharm Sci 95(11):2543–2552. https://doi.org/10.1002/jps.20744
Patil SP, Jain PD, Ghumatkar PJ, Tambe R, Sathaye S (2014) Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience 277:747–754. https://doi.org/10.1016/j.neuroscience.2014.07.046
Wang YW, He SJ, Feng X, Cheng J, Luo YT, Tian L, Huang Q (2017) Metformin: a review of its potential indications. Drug Des Dev Ther 11:2421–2429. https://doi.org/10.2147/DDDT.S141675
Arauz-Contreras J, Feria-Velasco A (1984) Monosodium-L-glutamate-induced convulsions—I. Differences in seizure pattern and duration of effect as a function of age in rats. Gen Pharmacol 15(5):391–395. https://doi.org/10.1016/0306-3623(84)90036-3
Peñafiel R, Cremades A, Monserrat F et al (1991) Monosodium glutamate induced convulsions in rats: influence of route of administration, temperature and age. Amino Acids 1:81–89. https://doi.org/10.1007/BF00808094
Hinzman JM, Thomas TC, Burmeister JJ, Quintero JE, Huettl P, Pomerleau F, Gerhardt GA, Lifshitz J (2010) Diffuse brain injury elevates tonic glutamate levels and potassium-evoked glutamate release in discrete brain regions at two days post-injury: an enzyme-based microelectrode array study. J Neurotrauma 27(5):889–899. https://doi.org/10.1089/neu.2009.1238
McEntee WJ, Crook TH (1993) Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology 111(4):391–401. https://doi.org/10.1007/BF02253527
Hanasand M, Omdal R, Norheim KB, Gøransson LG, Brede C, Jonsson G (2012) Improved detection of advanced oxidation protein products in plasma. Clin Chim Acta 413(9–10):901–906. https://doi.org/10.1016/j.cca.2012.01.038
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310. https://doi.org/10.1016/s0076-6879(78)52032-6
Jiang ZY, Hunt JV, Wolff SP (1992) Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem 202(2):384–389. https://doi.org/10.1016/0003-2697(92)90122-n
Yanar K, Atukeren P, Cebe T, Kunbaz A, Ozan T, Kansu AD, Durmaz S, Güleç V, Belce A, Aydın S, Çakatay U, Rizvi SI (2015) Ameliorative effects of testosterone administration on renal redox homeostasis in naturally aged rats. Rejuvenation Res 18(4):299–312. https://doi.org/10.1089/rej.2014.1640
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34(3):497–500
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Adamo AM, Zago MP, Mackenzie GG, Aimo L, Keen CL, Keenan A, Oteiza PI (2010) The role of zinc in the modulation of neuronal proliferation and apoptosis. Neurotox Res 17(1):1–14. https://doi.org/10.1007/s12640-009-9067-4
Seth R, Corniola RS, Gower-Winter SD, Morgan TJ Jr, Bishop B, Levenson CW (2015) Zinc deficiency induces apoptosis via mitochondrial p53- and caspase-dependent pathways in human neuronal precursor cells. J Trace Elem Med Biol 30:59–65. https://doi.org/10.1016/j.jtemb.2014.10.010
Abramov AY, Scorziello A, Duchen MR (2007) Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neuroscience 27(5):1129–1138. https://doi.org/10.1523/JNEUROSCI.4468-06.2007
Shinohe A, Hashimoto K, Nakamura K et al (2006) Increased serum levels of glutamate in adult patients with autism. Prog Neuropsychopharmacol Biol Psychiatry 30(8):1472–1477. https://doi.org/10.1016/j.pnpbp.2006.06.013
Murphy MP (1999) Nitric oxide and cell death. Biochem Biophys Acta 1411(2–3):401–414. https://doi.org/10.1016/s0005-2728(99)00029-8
Kirpichnikov D, McFarlane SI, Sowers JR (2002) Metformin: an update. Ann Intern Med 137(1):25–33. https://doi.org/10.7326/0003-4819-137-1-200207020-00009
Leng Y, Chuang DM (2006) Endogenous alpha-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. J Neuroscience 26(28):7502–7512. https://doi.org/10.1523/JNEUROSCI.0096-06.2006
Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM (2008) Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition. J Neuroscience 28(10):2576–2588. https://doi.org/10.1523/JNEUROSCI.5467-07.2008
Cross DA, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD (2001) Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J Neurochem 77(1):94–102. https://doi.org/10.1046/j.1471-4159.2001.t01-1-00251.x
Miranda ER, Dey CS (2004) Effect of chromium and zinc on insulin signaling in skeletal muscle cells. Biol Trace Elem Res 101(1):19–36. https://doi.org/10.1385/BTER:101:1:19
Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ (2004) Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Investig 113(11):1535–1549. https://doi.org/10.1172/JCI19906
Shin S, Wolgamott L, Yu Y, Blenis J, Yoon SO (2011) Glycogen synthase kinase (GSK)-3 promotes p70 ribosomal protein S6 kinase (p70S6K) activity and cell proliferation. Proc Natl Acad Sci USA 108(47):E1204–E1213. https://doi.org/10.1073/pnas.1110195108
Lynch CJ, Patson BJ, Goodman SA, Trapolsi D, Kimball SR (2001) Zinc stimulates the activity of the insulin- and nutrient-regulated protein kinase mTOR. Am J Physiol Endocrinol Metab 281(1):E25–E34. https://doi.org/10.1152/ajpendo.2001.281.1.E25
Huang XT, Li C, Peng XP et al (2017) An excessive increase in glutamate contributes to glucose-toxicity in β-cells via activation of pancreatic NMDA receptors in rodent diabetes. Sci Rep 7:44120. https://doi.org/10.1038/srep44120
Shen W, Taylor B, Jin Q et al (2015) Inhibition of DYRK1A and GSK3B induces human β-cell proliferation. Nat Commun 6:8372. https://doi.org/10.1038/ncomms9372
Domínguez RO, Pagano MA, Marschoff ER, González SE, Repetto MG, Serra JA (2014) Alzheimer disease and cognitive impairment associated with diabetes mellitus type 2: associations and a hypothesis. Neurologia 29(9):567–572. https://doi.org/10.1016/j.nrl.2013.05.006
Li DW, Liu ZQ, Chen W, Yao M, Li GR (2014) Association of glycogen synthase kinase-3β with Parkinson’s disease (review). Mol Med Rep 9(6):2043–2050. https://doi.org/10.3892/mmr.2014.2080
Khallaghi B, Safarian F, Nasoohi S, Ahmadiani A, Dargahi L (2016) Metformin-induced protection against oxidative stress is associated with AKT/mTOR restoration in PC12 cells. Life Sci 148:286–292. https://doi.org/10.1016/j.lfs.2016.02.024
Ouslimani N, Peynet J, Bonnefont-Rousselot D, Thérond P, Legrand A, Beaudeux JL (2005) Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism 54(6):829–834. https://doi.org/10.1016/j.metabol.2005.01.029
Zhao RR, Xu XC, Xu F, Zhang WL, Zhang WL, Liu LM, Wang WP (2014) Metformin protects against seizures, learning and memory impairments and oxidative damage induced by pentylenetetrazole-induced kindling in mice. Biochem Biophys Res Commun 448(4):414–417. https://doi.org/10.1016/j.bbrc.2014.04.130
Funding
This study was funded by the Istanbul University Scientific Research Projects. Project No: 27509.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to this research.
Conceived and designed the experiments and wrote the manuscript: AO, GS. Performed the experiments and analyzed the data: AO, KYO, KY, MM, MA, AC, BOK, OFS, UC, HU, GS. The corresponding author: AO. All authors have been involved in the drafting, critical revision, and final approval of the manuscript for publication. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics Approval
Ethical approval was obtained for the project from Istanbul University Animal Experiments Local Ethics Committee with the number 35980450–050.99. Guide for the Care and Use of Laboratory Animals of National Academy of Sciences was followed for caring animals during the experiment.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oruc, A., Oruc, K.Y., Yanar, K. et al. The Role of Glycogen Synthase Kinase-3β in the Zinc-Mediated Neuroprotective Effect of Metformin in Rats with Glutamate Neurotoxicity. Biol Trace Elem Res 202, 233–245 (2024). https://doi.org/10.1007/s12011-023-03667-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03667-3