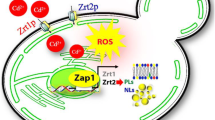
Abstract
Cadmium (Cd) is a toxic heavy metal mainly originating from industrial activities and causes environmental pollution. To better understand its toxicity and pollution remediation, we must understand the effects of Cd on living beings. Saccharomyces cerevisiae (budding yeast) is an eukaryotic unicellular model organism. It has provided much scientific knowledge about cellular and molecular biology in addition to its economic benefits. Effects associated with copper and zinc, sulfur and selenium metabolism, calcium (Ca2+) balance/signaling, and structure of phospholipids as a result of exposure to cadmium have been evaluated. In yeast as a result of cadmium stress, “mitogen-activated protein kinase,” “high osmolarity glycerol,” and “cell wall integrity” pathways have been reported to activate different signaling pathways. In addition, abnormalities and changes in protein structure, ribosomes, cell cycle disruption, and reactive oxygen species (ROS) following cadmium cytotoxicity have also been detailed. Moreover, the key OLE1 gene that encodes for delta-9 FA desaturase in relation to cadmium toxicity has been discussed in more detail. Keeping all these studies in mind, an attempt has been made to evaluate published cellular and molecular toxicity data related to Cd stress, and specifically published on S. cerevisiae.






Similar content being viewed by others
References
Ashraf MY, Roohi M, Iqbal Z, Ashraf M, Ozturk M, Gucel S (2015) Cadmium (Cd) and lead (Pb) induced inhibition in growth and alteration in some biochemical attributes and mineral accumulation in mung bean [Vigna radiata (L.) Wilczek]. Commun Soil Sci Plant Anal 47:405–413
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Environ Res Public Health 17(11):3782
Farooq M, Ullah A, Usman M, Siddique KH (2020) Application of zinc and biochar help to mitigate cadmium stress in bread wheat raised from seeds with high intrinsic zinc. Chemosphere 260:127652
El-Esawi MA, Elkelish A, Soliman M, Elansary HO, Zaid A, Wani SH (2020) Serratia marcescens BM1 enhances cadmium stress tolerance and phytoremediation potential of soybean through modulation of osmolytes, leaf gas exchange, antioxidant machinery, and stress-responsive genes expression. Antioxidants 9(1):43
Ghori NH, Ghori T, Hayat MQ, Imadi SR, Gul A, Altay V, Ozturk M (2019) Heavy metal stress and responses in plants. Int J Environ Sci Technol 16(3):1807–1828
Bertin G, Averbeck D (2006) Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 88:1549–1559
Chunhabundit R (2016) Cadmium exposure and potential health risk from foods in contaminated area, Thailand. Toxicological Research 32:65–72
Zhang H, Reynolds M (2019) Cadmium exposure in living organisms: a short review. Sci Total Environ 678:761–767
Goering PL, Waalkes MP, Klaassen CD (1994) Toxicology of metals, biochemical effects. Handbook of experimental pharmacology: toxicology of metals. Biochemical Effects. Springer, N Y 115:189–214
Hengstler JG, Bolm-Audorff U, Faldum A, Janssen K, Reifenrath M, Götte W, Gebhard S (2003) Occupational exposure to heavy metals: DNA damage induction and DNA repair inhibition prove co-exposures to cadmium, cobalt and lead as more dangerous than hitherto expected. Carcinogenesis 24:63–73
Zalups RK, Ahmad S (2003) Molecular handling of cadmium in transporting epithelia. Toxicol Appl Pharmacol 186:163–188
Bishak YK, Payahoo L, Osatdrahimi A, Nourazarian A (2015) Mechanisms of cadmium carcinogenicity in the gastrointestinal tract. Asian Pacific Journal Cancer Prevention 16:9–21
Larsson SC, Orsini N, Wolk A (2015) Urinary cadmium concentration and risk of breast cancer: a systematic review and dose-response meta-analysis. Am J Epidemiol 182:375–380
Inglot P, Lewinska A, Potocki L, Oklejewicz B, Tabecka-Lonczynska A, Koziorowski M, Wnuk M (2012) Cadmium-induced changes in genomic DNA-methylation status increase aneuploidy events in a pig Robertsonian translocation model. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 747:182–189
Xiao CL, Liu Y, Tu W, Xia YJ, Tian KM, Zhou X (2016) Research progress of the mechanisms underlying cadmium-induced carcinogenesis. Chinese Journal of Preventive Medicine 50:380–384
McMurray CT, Tainer JA (2003) Cancer, cadmium and genome integrity. Nat Genet 34:239–241
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18:321–336
Knight AS, Zhou EY, Francis MB (2015) Development of peptoid-based ligands for the removal of cadmium from biological media. Chem Sci 6:4042–4048
Li X, Jiang X, Sun J, Zhu C, Li X, Tian L, Bai W (2017) Cytoprotective effects of dietary flavonoids against cadmium-induced toxicity. Ann N Y Acad Sci 1398:5–19
Bánfalvi G (2011) Cellular effects of heavy metals. Springer
Tamás MJ, Labarre J, Toledano MB, Wysocki R (2006) Mechanisms of toxic metal tolerance in yeast BT. In: Tamas MJ, Martinoia E (eds) Molecular biology of metal homeostasis and detoxification: from microbes to man. Springer, Berlin, Heidelberg, pp 395–454
Huang X, Li Y, Pan J, Li M, Lai Y, Gao J, Li X (2016) RNA-Seq identifies redox balance related gene expression alterations under acute cadmium exposure in yeast. Environ Microbiol Rep 8:1038–1047
Li ZS, Lu YP, Zhen RG, Szczypka M, Thiele DJ, Rea PA (1997) A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc Natl Acad Sci U S A 94:42–47
Szczypka MS, Wemmie JA, Moye-Rowley WS, Thiele DJ (1994) A yeast metal resistance protein similar to human cystic fibrosis transmembrane conductance regulator (CFTR) and multidrug resistance- associated protein. J Biol Chem 269:22853–22857
Ghosh M, Shen J, Rosen BP (1999) Pathways of As (III) detoxification in Saccharomyces cerevisiae. Proc Natl Acad Sci 96:5001–5006
Gueldry O, Lazard M, Delort F, Dauplais M, Grigoras I, Blanquet S, Plateau P (2003) Ycf1p-dependent Hg(II) detoxification in Saccharomyces cerevisiae. Eur J Biochem 270:2486–2496
Lazard M, Ha-Duong NT, Mounié S, Perrin R, Plateau P, Blanquet S (2011) Selenodiglutathione uptake by the Saccharomyces cerevisiae vacuolar ATP-binding cassette transporter Ycf1p. FEBS J 278(21):4112–4121
Guerinot ML (2000) The ZIP family of metal transporters. Biochimica et Biophysica Acta (BBA)-Biomembranes 1465(1–2):190–198
Lazard M, Blanquet S, Fisicaro P, Labarraque G, Plateau P (2010) Uptake of selenite by Saccharomyces cerevisiae involves the high and low affinity orthophosphate transporters. J Biol Chem 285(42):32029–32037
Gomes DS, Fragoso LC, Riger CJ, Panek AD, Eleutherio ECA (2002) Regulation of cadmium uptake by Saccharomyces cerevisiae. Biochimica et Biophysica Acta (BBA)-General Subjects 1573(1):21–25
Adle DJ, Lee J (2008) Expressional control of a cadmium-transporting P1B-type ATPase by a metal sensing degradation signal. J Biol Chem 283:31460–31468
Adle DJ, Wei W, Smith N, Bies JJ, Lee J (2009) Cadmium-mediated rescue from ER-associated degradation induces expression of its exporter. Proc Natl Acad Sci 106:10189–10194
Adle DJ, Sinani D, Kim H, Lee J (2007) A cadmium-transporting P1B-type ATPase in yeast Saccharomyces cerevisiae. J Biol Chem 282:947–955
Smith N, Wei W, Zhao M, Qin X, Seravalli J, Kim H, Lee J (2016) Cadmium and secondary structure-dependent function of a degron in Pca1p cadmium exporter. Journal of Biological Chemistry jbc-M116
Wysocki R, Tamás MJ (2010) How Saccharomyces cerevisiae copes with toxic metals and metalloids. FEMS Microbiol Rev 34:925–951
Robinson NJ, Winge DR (2010) Copper metallochaperones. Annu Rev Biochem 79:537–562
Culotta VC, Yang M, O’Halloran TV (2006) Activation of superoxide dismutases: putting the metal to the pedal. Biochimica et Biophysica Acta (BBA)-Molecular Cell Res 1763:747–758
Pufahl RA, Singer CP, Peariso KL, Lin SJ, Schmidt PJ, Fahrni CJ, Culotta VC, Penner-Hahn JE, O’halloran TV (1997) Metal ion chaperone function of the soluble Cu (I) receptor Atx1. Science 278:853–856
Huffman DL, O’Halloran TV (2001) Function, structure, and mechanism of intracellular copper trafficking proteins. Annu Rev Biochem 70:677–701
Heo DH, Baek IJ, Kang HJ, Kim JH, Chang M, Kang CM, Yun CW (2012) Cd2+ binds to Atx1 and affects the physical interaction between Atx1 and Ccc2 in Saccharomyces cerevisiae. Biotechnol Lett 34:303–307
Wei W, Smith N, Wu X, Kim H, Seravalli J, Khalimonchuk O, Lee J (2014) YCF1-mediated cadmium resistance in yeast is dependent on copper metabolism and antioxidant enzymes. Antioxid Redox Signal 21:1475–1489
Fauchon M, Lagniel G, Aude JC, Lombardia L, Soularue P, Petat C, Labarre J (2002) Sulfur sparing in the yeast proteome in response to sulfur demand. Mol Cell 9:713–723
Vido K, Spector D, Lagniel G, Lopez S, Toledano MB, Labarre J (2001) A proteome analysis of the cadmium response in Saccharomyces cerevisiae. J Biol Chem 276:8469–8474
Jamieson D (2002) Saving sulfur. Nat Genet 31:228–230
Thomas D, Surdin-Kerjan Y (1997) Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 61:503–532
Flick K, Ouni I, Wohlschlegel JA, Capati C, McDonald WH, Yates JR, Kaiser P (2004) Proteolysis-independent regulation of the transcription factor Met4 by a single Lys 48-linked ubiquitin chain. Nat Cell Biol 6:634–641
Kaiser P, Flick K, Wittenberg C, Reed SI (2000) Regulation of transcription by ubiquitination without proteolysis. Cell 102:303–314
Rouillon A, Barbey R, Patton EE, Tyers M, Thomas D (2000) Feedback-regulated degradation of the transcriptional activator Met4 is triggered by the SCF(Met30) complex. EMBO J 19:282–294
Flick K, Raasi S, Zhang H, Yen JL, Kaiser P (2006) A ubiquitin-interacting motif protects polyubiquitinated Met4 from degradation by the 26S proteasome. Nat Cell Biol 8:509–515
Kaiser P, Su N-Y, Yen JL, Ouni I, Flick K (2006) The yeast ubiquitin ligase SCFMet30: connecting environmental and intracellular conditions to cell division. Cell Div 1:16
Patton EE, Peyraud C, Rouillon A, Surdin-Kerjan Y, Tyers M, Thomas D (2000) SCF(Met30)-mediated control of the transcriptional activator Met4 is required for the G(1)-S transition. EMBO J 19:1613–1624
Su NY, Flick K, Kaiser P (2005) The F-Box protein Met30 is required for multiple steps in the budding yeast cell cycle. Mol Cell Biol 25:3875–3885
Barbey R, Baudouin-Cornu P, Lee TA, Rouillon A, Zarzov P, Tyers M, Thomas D (2005) Inducible dissociation of SCF & lt; sup & gt; Met30</sup> ubiquitin ligase mediates a rapid transcriptional response to cadmium. EMBO J 24:521–532
Yen JL, Su NY, Kaiser P (2005) The yeast ubiquitin ligase SCFMet30 regulates heavy metal response. Mol Biol Cell 16:1872–1882
Flick K, Kaiser P (2011) Cellular mechanisms to respond to cadmium exposure: ubiquitin ligases. In: Banfalvi G (ed) Cellular effects of heavy metals. Springer, Berlin, pp 275–289
Zwolak I (2019) The role of selenium in arsenic and cadmium toxicity: an updated review of scientific literature. Biol Trace Elem Res 193:44–63. https://doi.org/10.1007/s12011-019-01691-w
Kieliszek M, Błażejak S, Bzducha-Wróbel A, Kurcz A (2016) Effects of selenium on morphological changes in Candida utilis ATCC 9950 yeast cells. Biol Trace Elem Res 169:387–393
Dauplais M, Lazard M, Blanquet S, Plateau P (2013) Neutralization by metal ions of the toxicity of sodium selenide. PLoS One 8(1):e54353
Kieliszek M, Błażejak S, Bzducha-Wróbel A, Kot AM (2019) Effect of selenium on lipid and amino acid metabolism in yeast cells. Biol Trace Elem Res 187:316–327
Kieliszek M, Błażejak S, Bzducha-Wróbel A, Kot AM (2019) Effect of selenium on growth and antioxidative system of yeast cells. Mol Biol Rep 46:1797–1808
Ismael MA, Elyamine AM, Moussa MG, Cai M, Zhao X, Hu C (2019) Cadmium in plants: uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 11:255–277. https://doi.org/10.1039/c8mt00247a
Pardo B, Crabbé L, Pasero P (2017) Signaling pathways of replication stress in yeast. FEMS Yeast Research 17(2):fow101
Chen S, Smolka MB, Zhou H (2007) Mechanism of Dun1 activation by Rad53 phosphorylation in Saccharomyces cerevisiae. J Biol Chem 282:986–995
Elledge SJ, Zhou Z, Allen JB, Navas TA (1993) DNA damage and cell cycle regulation of ribonucleotide reductase. Bioessays 15:333–339
Chabes A, Domkin V, Thelander L (1999) Yeast Sml1, a protein inhibitor of ribonucleotide reductase. J Biol Chem 274:36679–36683
Zhao X, Rothstein R (2002) The Dun1 checkpoint kinase phosphorylates and regulates the ribonucleotide reductase inhibitor Sml1. Proc Natl Acad Sci 99:3746–3751
Chabes A, Georgieva B, Domkin V, Zhao X, Rothstein R, Thelander L (2003) Survival of DNA damage in yeast directly depends on increased dNTP levels allowed by relaxed feedback inhibition of ribonucleotide reductase. Cell 112:391–401
Håkansson P, Hofer A, Thelander L (2006) Regulation of mammalian ribonucleotide reduction and dNTP pools after DNA damage and in resting cells. J Biol Chem 281:7834–7841
Andreson BL, Gupta A, Georgieva BP, Rothstein R (2010) The ribonucleotide reductase inhibitor, Sml1, is sequentially phosphorylated, ubiquitylated and degraded in response to DNA damage. Nucleic Acids Res 38:6490–6501
Huang M, Zhou Z, Elledge SJ (1998) The DNA replication and damage checkpoint pathways induce transcription by inhibition of the Crt1 repressor. Cell 94:595–605
Domki V, Thelander L, Chabes A (2002) Yeast DNA damage-inducible Rnr3 has a very low catalytic activity strongly stimulated after the formation of a cross-talking Rnr1/Rnr3 complex. J Biol Chem 277:18574–18578
Baek IJ, Kang HJ, Chang M, Choi ID, Kang CM, Yun CW (2012) Cadmium inhibits the protein degradation of Sml1 by inhibiting the phosphorylation of Sml1 in Saccharomyces cerevisiae. Biochem Biophys Res Commun 424:385–390
Muthukumar K, Nachiappan V (2010) Cadmium-induced oxidative stress in Saccharomyces cerevisiae. Indian J Biochem Biophys 47(6):383–387
Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Smeets K (2010) Cadmium stress: an oxidative challenge. BioMetals 23:927–940
Nair AR, DeGheselle O, Smeets K, Van Kerkhove E, Cuypers A (2013) Cadmium-induced pathologies: where is the oxidative balance lost (or not)? International Journal of Molecular Sciences:6116–6143
Valko MMHCM, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208
Choong G, Liu Y, Templeton DM (2014) Interplay of calcium and cadmium in mediating cadmium toxicity. Chem Biol Interact 211:54–65
Wang X, Yi M, Liu H, Han Y, Yi H (2016) Reactive oxygen species and Ca2+ are involved in cadmium-induced cell killing in yeast cells. Can J Microbiol 63:153–159
Wu L, Chen Y, Gao H, Yin J, Huang L (2016) Cadmium-induced cell killing in Saccharomyces cerevisiae involves increases in intracellular NO levels. FEMS Microbiology Letters 363(6):fnw032
Cyert MS, Philpott CC (2013) Regulation of cation balance in Saccharomyces cerevisiae. Genetics 193:677–713
Ruta LL, Popa VC, Nicolau I, Danet AF, Iordache V, Neagoe AD, Farcasanu IC (2014) Calcium signaling mediates the response to cadmium toxicity in Saccharomyces cerevisiae cells. FEBS Lett 588:3202–3212
Batiza AF, Schulz T, Masson PH (1996) Yeast respond to hypotonic shock with a calcium pulse. J Biol Chem 271:23457–23462
Matsumoto TK, Ellsmore AJ, Cessna SG, Low PS, Pardo JM, Bressan RA, Hasegawa PM (2002) An osmotically induced cytosolic Ca2+ transient activates calcineurin signaling to mediate ion homeostasis and salt tolerance of Saccharomyces cerevisiae. J Biol Chem 277:33075–33080
Kanzaki M, Nagasawa M, Kojima I, Sato C, Naruse K, Sokabe M, Iida H (1999) Molecular identification of a eukaryotic, stretch-activated nonselective cation channel. Science 285:882–886
Viladevall L, Serrano R, Ruiz A, Domenech G, Giraldo J, Barceló A, Ariño J (2004) Characterization of the calcium-mediated response to alkaline stress in Saccharomyces cerevisiae. J Biol Chem 279:43614–43624
Catterall WA (2000) Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol 16:521–555
Popa CV, Dumitru I, Ruta LL, Danet AF, Farcasanu IC (2010) Exogenous oxidative stress induces Ca2+ release in the yeast Saccharomyces cerevisiae. FEBS J 277:4027–4038
Cunningham KW, Fink GR (1994a) Ca2+ transport in Saccharomyces cerevisiae. J Exp Biol 196:157–166
Cunningham KW, Fink GR (1994b) Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J Cell Biol 124:351–363
Cunningham KW, Fink GR (1996) Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol 16:2226–2237
Miseta A, Kellermayer R, Aiello DP, Fu L, Bedwell DM (1999) The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett 451(2):132–136
Sorin A, Rosas G, Rao R (1997) PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J Biol Chem 272:9895–9901
Strayle J, Pozzan T, Rudolph HK (1999) Steady-state free Ca2+ in the yeast endoplasmic reticulum reaches only 10 μM and is mainly controlled by the secretory pathway pump Pmr1. The EMBO Journal 18:4733LP
Matheos DP, Kingsbury TJ, Ahsan US, Cunningham KW (1997) Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev 11:3445–3458
Stathopoulos AM, Cyert MS (1997) Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev 11:3432–3444
Locke EG, Bonilla M, Liang L, Takita Y, Cunningham KW (2000) A homolog of voltage-gated Ca(2+) channels stimulated by depletion of secretory Ca(2+) in yeast. Mol Cell Biol 20:6686–6694
Palmer CP, Zhou XL, Lin J, Loukin SH, Kung C, Saimi Y (2001) A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane. Proc Natl Acad Sci 98:7801–7805
Su Z, Zhou X, Loukin SH, Haynes WJ, Saimi Y, Kung C (2009) The use of yeast to understand TRP-channel mechanosensitivity. Pflügers Archiv-European Journal of Physiology 458(5):861–867
Rajakumar S, Bhanupriya N, Ravi C, Nachiappan V (2016) Endoplasmic reticulum stress and calcium imbalance are involved in cadmium-induced lipid aberrancy in Saccharomyces cerevisiae. Cell Stress and Chaperones 21(5):895–906
Rajakumar S, Ravi C, Nachiappan V (2016) Defect of zinc transporter ZRT1 ameliorates cadmium induced lipid accumulation in Saccharomyces cerevisiae. Metallomics 8:453–460
Muthukumar K, Nachiappan V (2013) Phosphatidylethanolamine from phosphatidylserine decarboxylase2 is essential for autophagy under cadmium stress in Saccharomyces cerevisiae. Cell Biochem Biophys 67:1353–1363
Jonikas MC, Collins SR, Denic V, Oh E, Quan EM, Schmid V, Weissman JS (2009) Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science 323:1693–1697
Thibault G, Shui G, Kim W, McAlister GC, Ismail N, Gygi SP, Ng DTW (2012) The membrane stress response buffers lethal effects of lipid disequilibrium by reprogramming the protein homeostasis network. Mol Cell 48:16–27
Martin CE, Oh C-S, Jiang Y (2007) Regulation of long chain unsaturated fatty acid synthesis in yeast. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids 1771:271–285
Stukey JE, McDonough VM, Martin CE (1989) Isolation and characterization of OLE1, a gene affecting fatty acid desaturation from Saccharomyces cerevisiae. J Biol Chem 264:16537–16544
Siso MIG, Becerra M, Maceiras ML, Vázquez ÁV, Cerdán ME (2012) The yeast hypoxic responses, resources for new biotechnological opportunities. Biotechnol Lett 34:2161–2173
Rajakumar S, Abhishek A, Selvam GS, Nachiappan V (2020) Effect of cadmium on essential metals and their impact on lipid metabolism in Saccharomyces cerevisiae. Cell Stress and Chaperones 25:19–33
Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2020) Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol Plant 168(2):345–360
Rajakumar S, Nachiappan V (2017) Lipid droplets alleviate cadmium induced cytotoxicity in Saccharomyces cerevisiae. Toxicology Research 6:30–41
Fang Z, Chen Z, Wang S, Shi P, Shen Y, Zhang Y, Huang Z (2017) Overexpression of OLE1 enhances cytoplasmic membrane stability and confers resistance to cadmium in Saccharomyces cerevisiae. Applied Environmental Microbiology 83:e02319–e02316
Huang Z, Yu Y, Fang Z, Deng Y, Shen Y, Shi P (2018) OLE1 reduces cadmium-induced oxidative damage in Saccharomyces cerevisiae. FEMS Microbiology Letters 365:fny193
Thompson JE, Froese CD, Madey E, Smith MD, Hong Y (1998) Lipid metabolism during plant senescence. Prog Lipid Res 37:119–141
Tsaluchidu S, Puri BK (2008) Fatty acids and oxidative stress. Ann General Psychiatry 7(Suppl 1):S86
Kwast KE, Burke PV, Staahl BT, Poyton RO (1999) Oxygen sensing in yeast: evidence for the involvement of the respiratory chain in regulating the transcription of a subset of hypoxic genes. Proc Natl Acad Sci 96(10):5446–5451
Muthukumar K, Rajakumar S, Sarkar MN, Nachiappan V (2011) Glutathione peroxidase3 of Saccharomyces cerevisiae protects phospholipids during cadmium-induced oxidative stress. Antonie Van Leeuwenhoek 99:761–771
Kudo N, Nakagawa Y, Waku K, Kawashima Y, Kozuka H (1991) Prevention by zinc of cadmium inhibition of stearoyl-CoA desaturase in rat liver. Toxicology 68:133–142
Shivapurkar N, Reddy J, Chaudhary PM, Gazdar AF (2003) Apoptosis and lung cancer: a review. J Cell Biochem 88:885–898
Rodríguez-Peña JM, García R, Nombela C, Arroyo J (2010) The high-osmolarity glycerol (HOG) and cell wall integrity (CWI) signalling pathways interplay: a yeast dialogue between MAPK routes. Yeast 27:495–502
Jiang L, Cao C, Zhang L, Lin W, Xia J, Xu H, Zhang Y (2014) Cadmium-induced activation of high osmolarity glycerol pathway through its Sln1 branch is dependent on the MAP kinase kinase kinase Ssk2, but not its paralog Ssk22, in budding yeast. FEMS Yeast Res 14(8):1263–1272
Lee J, Liu L, Levin DE (2018) Stressing out or stressing in: intracellular pathways for SAPK activation. Current Genetics 1-5
Metin M, Metin OK (2019) Cellular responses of Saccharomyces cerevisiae against arsenic. International Journal of Innovative Approaches in Science Research 3:41–52
Xiong B, Zhang L, Xu H, Yang Y, Jiang L (2015) Cadmium induces the activation of cell wall integrity pathway in budding yeast. Chem Biol Interact 240:316–323
Kamada Y, Jung US, Piotrowski J, Levin DE (1995) The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev 9:1559–1571
Sharma SK, Goloubinoff P, Christen P (2008) Heavy metal ions are potent inhibitors of protein folding. Biochem Biophys Res Commun 372:341–345
Tamás MJ, Sharma SK, Ibstedt S, Jacobson T, Christen P (2014) Heavy metals and metalloids as a cause for protein misfolding and aggregation. Biomolecules 4(1):252–267
Jacobson T, Priya S, Sharma SK, Andersson S, Jakobsson S, Tanghe R, Christen P (2017) Cadmium causes misfolding and aggregation of cytosolic proteins in yeast. Mol Cell Biol MCB-00490
Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI (2006) Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science 311:1471–1474
Holland S, Lodwig E, Sideri T, Reader T, Clarke I, Gkargkas K, Avery SV (2007) Application of the comprehensive set of heterozygous yeast deletion mutants to elucidate the molecular basis of cellular chromium toxicity. Genome Biol 8:268
Ibstedt S, Sideri TC, Grant CM, Tamás MJ (2014) Global analysis of protein aggregation in yeast during physiological conditions and arsenite stress. Biology Open 3:913–923
Plateau P, Saveanu C, Lestini R, Dauplais M, Decourty L, Jacquier A, Blanquet S, Lazard M (2017) Exposure to selenomethionine causes selenocysteine misincorporation and protein aggregation in Saccharomyces cerevisiae. Sci Rep 7:44761
Nikoleta GT, Daniel PR, Brown P (2012) The yeast rab GTPase Ypt1 modulates unfolded protein response dynamics by regulating the stability of HAC1 RNA. PLoS Genet 8(7):e1002862
Hetz C, Chevet E, Oakes SA (2015) Proteostasis control by the unfolded protein response. Nat Cell Biol 17:829–838
Kimata Y, Ishiwata-Kimata Y, Ito T, Hirata A, Suzuki T, Oikawa D, Kohno K (2007) Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins. J Cell Biol 179:75–86
Guerfal M, Ryckaert S, Jacobs P, Ameloot P, Van Craenenbroeck K, De Rycke R, Callewaert N (2010) The HAC1 gene from Pichia pastoris: characterization and effect of its overexpression on the production of secreted, surface displayed and membrane proteins. Microb Cell Factories 9(1):49
Rüegsegger U, Leber JH, Walter P (2001) Block of HAC1 mRNA translation by long-range base pairing is released by cytoplasmic splicing upon induction of the unfolded protein response. Cell 107:103–114
Cox JS, Walter P (1996) A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87:391–404
Gardarin A, Chédin S, Lagniel G, Aude J, Godat E, Catty P, Labarre J (2010) Endoplasmic reticulum is a major target of cadmium toxicity in yeast. Mol Microbiol 76:1034–1048
Le QG, Ishiwata-Kimata Y, Kohno K, Kimata Y (2016) Cadmium impairs protein folding in the endoplasmic reticulum and induces the unfolded protein response. FEMS Yeast Research 16(5):fow049
Goldberg AL (2003) Protein degradation and protection against misfolded or damaged proteins. Nature 426:895–899
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075
Ciechanover A (2003) The ubiquitin proteolytic system and pathogenesis of human diseases: a novel platform for mechanism-based drug targeting. Portland Press Limited
Glickman MH, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82:373–428
Shang F, Taylor A (2004) Function of the ubiquitin proteolytic pathway in the eye. Exp Eye Res 78:1–14
Shabek N, Herman-Bachinsky Y, Ciechanover A (2009) Ubiquitin degradation with its substrate, or as a monomer in a ubiquitination-independent mode, provides clues to proteasome regulation. Proc Natl Acad Sci 106:11907–11912
Hershko A, Ciechanover A (1998) The ubiquitin system. Annual Reviews 4139 El Camino Way, PO Box 10139, Palo Alto, CA 94303-0139, USA
Nijman SMB, Luna-Vargas MPA, Velds A, Brummelkamp TR, Dirac AMG, Sixma TK, Bernards R (2005) A genomic and functional inventory of deubiquitinating enzymes. Cell 123:773–786
Wilkinson KD (2000) Ubiquitination and deubiquitination: targeting of proteins for degradation by the proteasome. Semin Cell Dev Biol 11:141–148
Lata S, Mishra R, Banerjea AC (2018) Proteasomal degradation machinery: favorite target of hiv-1 proteins. Front Microbiol 9:2738
Jungmann J, Reins HA, Schobert C, Jentsch S (1993) Resistance to cadmium mediated by ubiquitin-dependent proteolysis. Nature 361:369–371
Baudouin-Cornu P, Labarre J (2006) Regulation of the cadmium stress response through SCF-like ubiquitin ligases: comparison between Saccharomyces cerevisiae, Schizosaccharomyces pombe and mammalian cells. Biochimie 88:1673–1685
Laferté A, Favry E, Sentenac A, Riva M, Carles C, Chédin S (2006) The transcriptional activity of RNA polymerase I is a key determinant for the level of all ribosome components. Genes Dev 20:2030–2040
Rudra D, Warner JR (2004) What better measure than ribosome synthesis? Genes Dev 18:2431–2436
Jin YH, Dunlap PE, McBride SJ, Al-Refai H, Bushel PR, Freedman JH (2008) Global transcriptome and deletome profiles of yeast exposed to transition metals. PLoS Genet 4:e1000053
Wang S, Shi X (2001) Molecular mechanisms of metal toxicity and carcinogenesis. Mol Cell Biochem 222(1–2):3–9
Zhou L, Le Roux G, Ducrot C, Chedin S, Labarre J, Riva M, Carles C (2013) Repression of class I transcription by cadmium is mediated by the protein phosphatase 2A. Nucleic Acids Res 41:6087–6097
Author information
Authors and Affiliations
Contributions
The conception and design of the study: M.O., M.M., and V.A. Acquisition of data, analysis, and interpretation of data: M.O., M.M., V.A., B.T.U., A.K., A.G., M.H., K.N., T.K., and P.G.C. Drafting the article: M.O., M.M., V.A., A.K., A.G., M.H., K.N., and T.K. Revising the article critically for important intellectual content: M.O., V.A., A.K., A.G., M.H., K.N., and L.D. Final approval of the version to be submitted: M.O., V.A., and L.D.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ozturk, M., Metin, M., Altay, V. et al. Molecular Biology of Cadmium Toxicity in Saccharomyces cerevisiae. Biol Trace Elem Res 199, 4832–4846 (2021). https://doi.org/10.1007/s12011-021-02584-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02584-7