Abstract
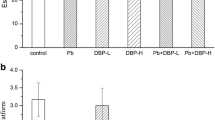
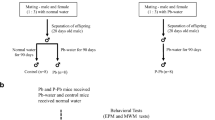
Lead and di-2-ethylhexyl phthalate (DEHP) are widely distributed in the environment, and their neurotoxicity has caused a widespread concern. The complexity of environmental exposure provides the possibility of their combined exposure. The present study aims to describe a joint neurotoxicity and clarify the potential mechanism after combined exposure to lead and DEHP. A 2 × 3 factorial design was used to analyze either single effects or their interaction by a subchronic lead and DEHP exposure model of the male weaning rats. Similar to the previous study, lead or DEHP single exposure showed an increased neurotoxicity. Interestingly, our neurobehavioral test showed the rats in the combined exposure groups had a better ability of learning and memory compared with the single-exposure ones. It seemed to reflect an antagonism joint action in neurotoxicity after combined exposure. The content of dehydroepiandrosterone (DHEA) in serum and the mRNA level of brain-derived neurotrophic factor (Bdnf) in the hippocampus showed a similar trend to the ability of learning and memory. However, there was insufficient evidence to support the joint action on some indexes of oxidative stress such as malondialdehyde (MDA), the ratio of reduced glutathione(GSH) to oxidized glutathione(GSSG), γglutamylcysteine synthetase (γ-GCS), glutathione-s transferase (GST), and nuclear factor E2-related factor 2 (Nrf2) mRNA expression in the hippocampus. In a word, our current study reminded a unique antagonism joint action of neurotoxicity after combined exposure to lead and DEHP, which may contribute to understanding some shallow mechanism of the joint toxicity due to the complexity of environmental pollutant exposure.




Similar content being viewed by others
References
Aksu D, Didin M, Kayikci F (2012) The protective role of polyphenols on blood cells in rats exposed to lead Rolul protectiv al polifenolilor asupra celulelor sanguine la şobolanii expuşi la plumb
Murata K, Iwata T, Dakeishi M, Karita K (2009) Lead toxicity: does the critical level of lead resulting in adverse effects differ between adults and children? J Occup Health 51(1):1–12
Rosner D, Markowitz G (2007) The politics of lead toxicology and the devastating consequences for children. Am J Ind Med 50(10):740–756. https://doi.org/10.1002/ajim.20435
Saripinar Aksu D, Saglam YS, Aksu T (2016) The investigation of neuroprotective effects of pomegranate juice on oxidative damage in brain caused by lead in rats. Eurasian J Vet Sci 32(4):255–255. https://doi.org/10.15312/EurasianJVetSci.2016422397
Aksu DS, Saglam YS, Yildirim S, Aksu T (2017) Effect of pomegranate (Punica granatum L.) juice on kidney, liver, heart and testis histopathological changes, and the tissues lipid peroxidation and antioxidant status in lead acetate-treated rats. Cell Mol Biol (Noisy-le-grand) 63(10):33–42. https://doi.org/10.14715/cmb/2017.63.10.5
Ozkaya A, Sahin Z, Kuzu M, Saglam YS, Ozkaraca M, Uckun M, Yologlu E, Comakli V, Demirdag R, Yologlu S (2018) Role of geraniol against lead acetate-mediated hepatic damage and their interaction with liver carboxylesterase activity in rats. Arch Physiol Biochem 124(1):80–87. https://doi.org/10.1080/13813455.2017.1364772
Krigman MR, Hogan EL (1974) Effect of lead intoxication on the postnatal growth of the rat nervous system. Environ Health Perspect 7:187–199
Chen A, Dietrich KN, Ware JH, Radcliffe J, Rogan WJ (2005) IQ and blood lead from 2 to 7 years of age: are the effects in older children the residual of high blood lead concentrations in 2-year-olds? Environ Health Perspect 113(5):597–601
Ordemann JM, Austin RN (2016) Lead neurotoxicity: exploring the potential impact of lead substitution in zinc-finger proteins on mental health. Metallomics 8(6):579–588. https://doi.org/10.1039/c5mt00300h
Lu X, Jin C, Yang J, Liu Q, Wu S, Li D, Guan Y, Cai Y (2013) Prenatal and lactational lead exposure enhanced oxidative stress and altered apoptosis status in offspring rats’ hippocampus. Biol Trace Elem Res 151(1):75–84. https://doi.org/10.1007/s12011-012-9531-5
Zhao Q, Slavkovich V, Zheng W (1998) Lead exposure promotes translocation of protein kinase C activities in rat choroid plexus in vitro but not in vivo. Toxicol Appl Pharmacol 149(1):99–106
Guilarte TR, McGlothan JL (1998) Hippocampal NMDA receptor mRNA undergoes subunit specific changes during developmental lead exposure. Brain Res 790(1–2):98–107
Kamrin MA (2009) Phthalate risks, phthalate regulation, and public health: a review. J Toxicol Environ Health B Crit Rev 12(2):157–174. https://doi.org/10.1080/10937400902729226
Joffe M (2001) Are problems with male reproductive health caused by endocrine disruption? Occup Environ Med 58(4):281–287 quiz 287–288, 260
Gartner S, Balski M, Koch M, Nehls I (2009) Analysis and migration of phthalates in infant food packed in recycled paperboard. J Agric Food Chem 57(22):10675–10681. https://doi.org/10.1021/jf902683m
Beliles R, Salinas JA, Kluwe WM (1989) A review of di(2-ethylhexyl)phthalate (DEHP) risk assessments. Drug Metab Rev 21(1):3–12. https://doi.org/10.3109/03602538909029952
Poon R, Lecavalier P, Mueller R, Valli VE, Procter BG, Chu I (1997) Subchronic oral toxicity of di-n-octyl phthalate and di(2-ethylhexyl) phthalate in the rat. Food Chem Toxicol 35(2):225–239
Zarean M, Keikha M, Poursafa P, Khalighinejad P, Amin M, Kelishadi R (2016) A systematic review on the adverse health effects of di-2-ethylhexyl phthalate. Environ Sci Pollut Res Int 23(24):24642–24693. https://doi.org/10.1007/s11356-016-7648-3
Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, Miodovnik A, Wolff MS (2009) Prenatal phthalate exposure and performance on the neonatal behavioral assessment scale in a multiethnic birth cohort. Neurotoxicology 30(4):522–528. https://doi.org/10.1016/j.neuro.2009.04.001
Cho SC, Bhang SY, Hong YC, Shin MS, Kim BN, Kim JW, Yoo HJ, Cho IH, Kim HW (2010) Relationship between environmental phthalate exposure and the intelligence of school-age children. Environ Health Perspect 118(7):1027–1032
Kim BN, Cho SC, Kim Y, Shin MS, Yoo HJ, Kim JW, Yang YH, Kim HW, Bhang SY, Hong YC (2009) Phthalates exposure and attention-deficit/hyperactivity disorder in school-age children. Biol Psychiatry 66(10):958–963. https://doi.org/10.1016/j.biopsych.2009.07.034
Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I (1999) Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol 1(3):165–170. https://doi.org/10.1038/11086
McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA (1996) Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell 87(7):1339–1349
Dzhekova-Stojkova S, Bogdanska J, Stojkova Z (2001) Peroxisome proliferators: their biological and toxicological effects. Clin Chem Lab Med 39(6):468–474. https://doi.org/10.1515/cclm.2001.076
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1(2):848–858
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55(4):611–622. https://doi.org/10.1373/clinchem.2008.112797
Jett DA, Kuhlmann AC, Farmer SJ, Guilarte TR (1997) Age-dependent effects of developmental lead exposure on performance in the Morris water maze. Pharmacol Biochem Behav 57(1–2):271–279
Arcadi FA, Costa C, Imperatore C, Marchese A, Rapisarda A, Salemi M, Trimarchi GR, Costa G (1998) Oral toxicity of bis(2-ethylhexyl) phthalate during pregnancy and suckling in the long-Evans rat. Food Chem Toxicol 36(11):963–970
Meltzer D, Martinez-Arguelles DB, Campioli E, Lee S, Papadopoulos V (2015) In utero exposure to the endocrine disruptor di(2-ethylhexyl) phthalate targets ovarian theca cells and steroidogenesis in the adult female rat. Reprod Toxicol 51:47–56. https://doi.org/10.1016/j.reprotox.2014.12.005
Braun JM, Bellinger DC, Hauser R, Wright RO, Chen A, Calafat AM, Yolton K, Lanphear BP (2017) Prenatal phthalate, triclosan, and bisphenol a exposures and child visual-spatial abilities. Neurotoxicology 58:75–83. https://doi.org/10.1016/j.neuro.2016.11.009
Smith CA, Holahan MR (2014) Reduced hippocampal dendritic spine density and BDNF expression following acute postnatal exposure to di(2-ethylhexyl) phthalate in male long Evans rats. PLoS One 9(10):e109522. https://doi.org/10.1371/journal.pone.0109522
Dai Y, Yang Y, Xu X, Hu Y (2015) Effects of uterine and lactational exposure to di-(2-ethylhexyl) phthalate on spatial memory and NMDA receptor of hippocampus in mice. Horm Behav 71:41–48. https://doi.org/10.1016/j.yhbeh.2015.03.008
Fu H, Chen Z, Josephson L, Li Z, Liang SH (2018) Positron emission tomography (pet) ligand development for ionotropic glutamate receptors: challenges and opportunities for radiotracer targeting N-methyl-d-aspartate (NMDA), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate receptors. J Med Chem. https://doi.org/10.1021/acs.jmedchem.8b00714
Neal AP, Stansfield KH, Worley PF, Thompson RE, Guilarte TR (2010) Lead exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: potential role of NMDA receptor-dependent BDNF signaling. Toxicol Sci 116(1):249–263. https://doi.org/10.1093/toxsci/kfq111
Cao XJ, Huang SH, Wang M, Chen JT, Ruan DY (2008) S-adenosyl-L-methionine improves impaired hippocampal long-term potentiation and water maze performance induced by developmental lead exposure in rats. Eur J Pharmacol 595(1–3):30–34. https://doi.org/10.1016/j.ejphar.2008.07.061
Wu Y, Li K, Zuo H, Yuan Y, Sun Y, Yang X (2014) Primary neuronal-astrocytic co-culture platform for neurotoxicity assessment of di-(2-ethylhexyl) phthalate. J Environ Sci 26(5):1145–1153. https://doi.org/10.1016/s1001-0742(13)60504-5
Funding
This study was supported by the National Natural Science Foundation of China 81773470 and the Provincial Natural Science Foundation of Liaoning 20170540991.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval for all animals involved in this study was from the Institutional Animal Care and Use Committee of China Medical University.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, L., Li, H., Qu, P. et al. An Antagonism Joint Action of Lead and Di-2-Ethylhexyl Phthalate Explains an Improved Ability of Learning and Memory after Combined Exposure in Weaning Rats. Biol Trace Elem Res 191, 126–134 (2019). https://doi.org/10.1007/s12011-018-1586-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1586-5