Abstract
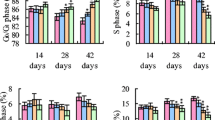
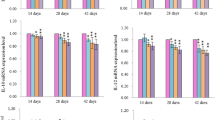
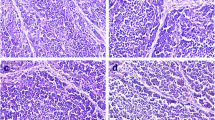
The purpose of this 42-day study was to investigate the apoptosis in the bursa of Fabricius induced by different levels of dietary vanadium. A total of 420 1-day-old avian broilers were divided into 6 groups in which there were 7 replicates in each group and 10 broilers in each replicate and fed on a corn–soybean basal diet as control diet (vanadium 0.073 mg/kg) or the same diet amended to contain 5, 15, 30, 45, and 60 mg/kg vanadium supplied as ammonium metavanadate (NH4VO3). Ultrastructurally, mitochondrial injury and increased numbers of apoptotic cells with condensed nuclei were observed in the 30, 45, and 60 mg/kg groups. As measured by flow cytometry, the percentages of apoptotic lymphocytes were significantly increased in the 15-, 30-, 45-, and 60-mg/kg groups when compared with those of control group. Meanwhile, the terminal deoxynucleotidyl transferase 2′-deoxyuridine 5′-triphosphate nick end-labeling assay showed that there were increased numbers of apoptotic cells in the 30-, 45-, and 60-mg/kg groups. Immunohistochemical tests showed increased numbers of positive cells under Bax and caspase-3 protein detection and decreased Bcl-2 protein in the 15-, 30-, 45-, and 60-mg/kg groups. The vanadium content of the bursa was found to be significantly increased in the 30-, 45-, and 60-mg/kg groups. These results suggested that dietary vanadium in excess of 15 mg/kg could cause lymphocyte apoptosis in the bursa of Fabricius and impact humoral immunity in broilers. Lymphocyte apoptosis in the bursa induced by high levels of dietary vanadium is associated with mitochondrial injury and changes in levels of apoptogenic proteins, such as Bcl-2, Bax, and caspase-3.






Similar content being viewed by others
References
French RJ, Jones PJH (1993) Role of vanadium in nutrition: metabolism, essentiality and dietary consideration. Life Sci 52(4):339–346
Nielsen FH (1990) New essential trace elements for the life sciences. Biol Trace Elem Res 26–27(1):599–611
Duckworth WC, Solomon SS, Liepnieks J et al (1998) Insulin-like efects of vanadium in isolated rat adipocytes. Endocrinology 122:2285–2289
Crans DC, Smee JJ, Gaidamauskas E et al (2004) The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem Rev 104(2):849–902
Domingo JL (2002) Vanadium and tungsten derivatives as antidiabetic agents. Biol Trace Elem Res 88(2):97–112
Norgaard A, Kjeldsev K, Hansen O et al (1983) A simple and rapid method for the determination of the number of H3-ouabain binding sites in biopsies of skeletal muscle. Biochem Biophys Res Commun 111(1):319–325
Bahdan RN (1984) Mechanisms of action of vanadium. Annu Rev Pharmacol Toxicol 24:501–507
Schmitz W, Scholz H (1982) Effect of vanadium in the +5, +4 and +3 oxidation states on cardiac force of contraction, adenylate cyclase and (Na+, K+)-ATPase activity. Biochem Pharmacol 31(23):3853–3860
Bishayee A, Chatterjee M (1995) Time course effects of vanadium supplement on cytosolic reduced glutathione level and glutathione S-transferase activity. Biol Trace Elem Res 48(3):275–285
Liochev SI, Fridovich I (1990) Vanadate-stimulated oxidation of NAD (P) H in the presence of biological membranes and other sources of O −2 . Arch Biochem Biophys 279(1):1–7
Feng BM, Sun SL, Yang CQ (2008) Effects of vanadium on GSH-Px activity in muscle of mice. Prog Vet Med 29(2):42–44
Biswajit M, Balaram P, Sushmita M et al (2004) Vanadium—an element of atypical biological significance. Toxicol Lett 150:135–143
Deng Y, Cui HM, Peng X et al (2011) Dietary vanadium induces oxidative stress in the intestine of broilers. Biol Trace Elem Res. doi:10.1007/s12011-011-9163-1
Liu J, Cui HM, Liu X et al (2011) Dietary high vanadium causes oxidative damage-induced renal and hepatic toxicity in broilers. Biol Trace Elem Res. doi:10.1007/s12011-011-9185-8
Cui W, Cui HM, Peng X et al (2010) The changes of relative weight, lesions, and cell cycle of bursa of Fabricius induced by dietary excess vanadium in broilers. Biol Trace Elem Res. doi:10.1007/s12011-010-8832-9
Cui W, Cui HM, Peng X et al (2011) Effect of vanadium on the subset and proliferation of peripheral blood T-cells and serum IL-2 content in broilers. Biol Trace Elem Res 141:192–199
Deng J, Deng Y, Cui HM, Peng X et al (2011) Effect of dietary vanadium on cecal tonsil T-cell subsets and IL-2 contents in broilers. Biol Trace Elem Res. doi:10.1007/s12011-011-9018-9
Tompson CB (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267:1456–1462
Huang C, Zhang Z, Diog M et al (2000) Vanadate induces p53 transactivation through hydrogen peroxide and c8uses apoptosis. Biol Chem 275:32516–32522
Cortizo AM, Caporossi M, Lettieri G (2000) Vanadate-induced nitric oxide production: role in osteoblast growth and differentiation. Eur J Pharmacol 400:279–285
Seheving LA, Thomas JR, Zhang L (1999) Regulation of intestinal tyrosine phosphorylation and programmed cell death by peroxo vanadate. Am J Physiol 277:572–579
Peng X, Cui Y, Cui W, Deng J, Cui HM (2009) The decrease of relative weight, lesions, and apoptosis of bursa of Fabricius induced by excess dietary selenium in chickens. Biol Trace Elem Res 131(1):33–42
Wang JM, Xiao BL, Zheng JW et al (2007) Effect of targeted magnetic nanoparticles containing 5-FU on expression of bcl-2, bax and caspase-3 in nude mice with transplanted human liver cancer. World J Gastroenterol 13(23):3171–3175
Lee JH, Jung JY, Jeong YJ et al (2008) Involvement of both mitochondrial- and death receptor-dependent apoptotic pathways regulated by Bcl-2 family in sodium fluoride-induced apoptosis of human gingival fibroblasts. Toxicol 243:340–347
Roset R, Ortet L, Gil-Gomez G (2007) Role of Bcl-2 family members on apoptosis: what we have learned from knock-out mice. Front Biosci 12(20):4722–4730
Palmer AM, Greengrass PM, Cavali AD (2000) The role of mitochondria in apoptosis. Drug News Perspect 13(6):378–384
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivio with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74(4):609–619
Rana SVS (2008) Metals and apoptosis: recent development. J Trace Elem Med Bio 22(4):262–284
Sit KH, Paramanant HR, Bay BH et al (1996) Induction of vanadium accumulation and nuclear sequestration causing cell suicide in human liver cells. Cell Mol Life Sci 52(8):778–785
Green DR (2000) Apoptotic pathways: paper wraps stone blunts scissors. Cell 102:1–4
Zamzami N, Marchetti P, Castedo M (1995) Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med 181:161
Altamirano-Lozano M, Alvarez-Barrera L, Basurto-Alcántara F et al (1996) Reprotoxic and genotoxic studies of vanadium pentoxide in male mice. Teratog Carcinog Mutagen 16(1):7–17
Shi XL, Jang HG, Mao Y et al (1996) Vanadium(IV)-mediated free radical generation and related 2′-deoxyguanosine hydroxylation and DNA damage. Toxicol 106(1–3):27–38
Acknowledgments
The study was supported by the program for Changjiang scholars and innovative research team in a university (IRT 0848) and the key program of education department and scientific department of Sichuan Province (09ZZ017).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, W., Cui, H., Peng, X. et al. Dietary Vanadium Induces Lymphocyte Apoptosis in the Bursa of Fabricius of Broilers. Biol Trace Elem Res 146, 59–67 (2012). https://doi.org/10.1007/s12011-011-9215-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-9215-6