Abstract
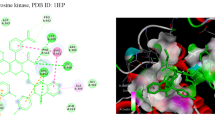
Two new heteroleptic Ru(II) polypyridyl complexes, [Ru(bpy)2(B)]Cl2 (RBB) (bpy = 2,2′-bipyridine and B = 4,4′-bis(benzimidazolyl)-2,2′-bipyridine) and [Ru(phen)2(B)]Cl2 (RPB), were synthesized, and the structural features were confirmed by the analytical and spectral tools such as FT-IR, 1H-NMR, and UV–Visible spectroscopy. We have explored the possibility of improving the selectivity of cytotoxic Ru(II) complex and their preliminary biological evaluation against MCF-7 and MG-63 cell lines and clinical pathogens. The results of the antimicrobial screening show that the ligand and complexes have a range of abilities against the species of bacteria and fungi that were tested. The anti-inflammatory activity of the compounds was found to be in the range of 30–75%. Molecular docking study was performed for these ligand and complexes to evaluate and analyze the anti-lymphoma cancer activity. Molecular docking score and the rank revealed the bonding affinity towards the site of interaction of the oncoprotein anaplastic lymphoma kinase (ALK).










Similar content being viewed by others
Data Availability
The data available and/or analyzed in this the current work is available from the corresponding author on request.
References
Cao, B., Bray, F., Ilbawi, A., & Soerjomataram, I. (2018). The Lancet. Global Health., 6, 1288–1296.
Arbyn, M., Weiderpass, E., de Bruni, L., Sanjosé, S., Saraiya, M., Ferlay, J., & Bray, F. (2020). The Lancet. Global Health., 8, 191–203.
Lionta, E., Spyrou, G. K., Vassilatis, D. & Cournia, Z. (2014). Current Topics In Medicinal Chemistry, 14, 1923–1938.
Bennett, J. E., Stevens, G. A., Mathers, C. D., Bonita, R., Rehm, J., Kruk, M. E., & Ezzati, M. (2018). The lancet., 392, 1072–1088.
van Rijt, S. H., & Sadler, P. J. (2009). Drug Discovery Today, 14, 1089–1097.
Ghosh, S. (2019). Bioorganic Chemistry, 88, 102925.
Dasari, S. & Tchounwou, P. B. (2014). European Journal of Pharmacology, 740, 364–378.
Florea, A. M., & Büsselberg, D. (2011). Cancers, 3, 1351–1371.
Kaim, W., Schwederski, B., & Klein, A. (2013). Bioinorganic chemistry–inorganic elements in the chemistry of life: An introduction and guide. John Wiley and Sons.
Hernández Romero, D., Rosete Luna, S., López Monteon, A., Chávez Piña, A., Pérez Hernández, N., Marroquín Flores, J., & Colorado Peralta, R. (2021). Coordination Chemistry Reviews, 439, 213930.
Henary, M., Kananda, C., Rotolo, L., Savino, B., Owens, E. A., & Cravotto, G. (2020). RSC Advances., 10, 14170–14197.
Zha, G. F., Rakesh, K. P., Manukumar, H. M., Shantharam, C. S., & Long, S. (2019). European Journal of Medicinal Chemistry., 162, 465–494.
Bansal, Y. & Silakari, O. (2012). Bioorganic and Medicinal Chemistry, 20, 6208–6236.
Wang, X., Ling, N., Che, Q. T., Zhang, Y. W., Yang, H. X., Ruan, Y., & Zhao, T. T. (2019). Inorganic Chemistry Communications, 105, 97-101.
Elsayed, S. A., Harrypersad, S., Sahyon, H. A., El-Magd, M. A., & Walsby, C. J. (2020). Molecules, 25, 4284.
Ghanghas, P., Choudhary, A., Kumar, D., & Poonia, K. (2021). Inorganic Chemistry Communications, 130, 108710.
Bruijnincx, P. C. & Sadler, P. J. (2008). Current Opinion in Chemical Biology, 12, 197-206.
Ortega, E., Vigueras, G., Ballester, F. J., & Ruiz, J. (2021). Coordination Chemistry Reviews, 446, 214129.
Kundu, B. K. & Mukhopadhyay, S. (2021). Coordination Chemistry Reviews, 448, 214169.
Pete, S., Roy, N., Kar, B., & Paira, P. (2022). Coordination Chemistry Reviews, 460, 214462.
Lenis Rojas, O. A., Cabral, R., Carvalho, B., Friaes, S., Roma Rodrigues, C., Fernandez, J. A., & Royo, B. (2021). Inorganic Chemistry, 60, 8011–8026.
Tan, C. P., Zhong, Y. M., Ji, L. N., & Mao, Z. W. (2021). Chemical Science, 12, 2357-2367.
Machado, J. F., Correia, J. D., & Morais, T. S. (2021). Molecules, 26, 3153.
Maikoo, S., Chakraborty, A., Vukea, N., Dingle, L. M. K., Samson, W. J., de la Mare, J. A., & Booysen, I. N. (2021). Journal of Biomolecular Structure and Dynamics, 39, 4077-4088.
Takita, J. (2017). Cancer science. 108, 1913-1920.
Morris, S. W., Naeve, C., Mathew, P., James, P. L., Kirstein, M. N., Cui, X., & Witte, D. P. (1997). Oncogene. 14, 2175-2188
Roskoski Jr, R. (2013). Pharmacological research. 68, 68-94.
Chiarle, R., Voena, C., Ambrogio, C., Piva, R., & Inghirami, G. (2008). Nature Reviews Cancer, 8, 11-23.
Swarnalatha, K., Rathnamala, P., Babu, A. A., & Bhuvanesh, N. (2016). Journal of Structural Chemistry, 57, 1554-1560.
Nongpiur, C. G. L., Tripathi, D. K., Poluri, K. M., Rawat, H., & Kollipara, M. R. (2022). Journal of Chemical Sciences, 134, 1-14.
Nongpiur, C. G. L., Ghate, M. M., Tripathi, D. K., Poluri, K. M., Kaminsky, W., & Kollipara, M. R. (2022). Journal of Organometallic Chemistry, 957, 122148.
Gur’eva, Y. A., Zaleyskaya,O. A., Shevchenko, O. G., Slepukhin, P. A., Makarov, V. A., & Kuchin, A. V. (2022). RSC Advances, 128841–8851.
Ruberte, A. C., Ramos Inza, S., Aydillo, C., Talavera, I., Encío, I., Plano, D., & Sanmartín, C. (2020). Antioxidants, 9, 55.
Mumtaz, A., Mahmud, T., Khalid, M., Khan, H., Sadia, A., Samra, M. M., & Basra, M. A. R. (2020). Journal of Pharmaceutical Innovation, 1–9.
Cassiano, N. M., Barreiro, J. C., Moraes, M. C., Oliveira, R. V., & Cass, Q. B. (2009). Bioanalysis, 1, 577-594.
Ruiz, M. C., Kljun, J., Turel, I., Di Virgilio, A. L., & León, I. E. (2019). Metallomics, 11, 666-675.
Jiang, G. B., Xie, Y. Y., Lin, G. J., Huang, H. L., Liang, Z. H., & Liu, Y. J. (2013). Journal of Photochemistry and Photobiology B: Biology, 129, 48-56.
Vijayakumar, V., Prabakaran, A., Radhakrishnan, N., Muthu, S., Rameshkumar, C., & Paulraj, E. I. (2019). Journal of Molecular Structure, 1179, 325-335.
Balaji, T. E, Tanaya Das, H, & Maiyalagan, T. (2021). ChemElectroChem, 8, 1723-1746.
Webb, T. R., Slavish, J., George, R. E., Look, A. T., Xue, L., Jiang, Q., & Morris, S. W. (2009). Expert review of anticancer therapy. 9, 331-356.
Acknowledgement
The authors are thankful to Trichy Research Institute of Biotechnology PVT. Ltd. for helping in carrying out the biological studies. We are grateful to Dr. E. Angel Jemima for her contribution in the study of biological activities of the synthesized metal complexes.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the design and the study. The complexes are synthesized and the complete experimental part and biological studies are carried out by Kamaraj Karthick. Muthukumar Abinaya has helped in the compilation and analysis of the biological part. Dr. Thangaraj Shankar has contributed in the complete characterization of the ligand and metal complexes. Kalaiyar Swarnalatha is the team supervisor and she is the sole responsible (draft and the compilation) for the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karthick, K., Abinaya, M., Shankar, T. et al. In Vitro and In Silico Screening of Benzimidazole-Based Ruthenium(II) Complexes as Potent ALK Inhibitor for Cancer Prevention. Appl Biochem Biotechnol 195, 7397–7413 (2023). https://doi.org/10.1007/s12010-023-04435-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04435-8