Abstract
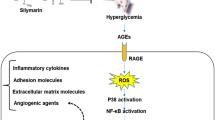
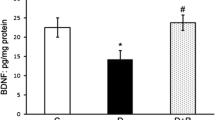
Diabetic retinopathy (DR) is a diabetes mellitus (DM) complication that causes visual acuity impairment and loss of sight in the working population, mainly in developed countries. According to the WHO, DR accounts for 5% of the world’s 37 million blind people. The prevalence of diabetic retinopathy was highest in Africa, followed by North America and the Caribbean and South and Central America. Hyperglycemia can generate excessive ROS that activates multiple pathways, which can damage the cells. Oxidative stress and inflammatory process are intricate in the DR pathological mechanism. Bilobalide is the main bioactive compound isolated from the Ginkgo biloba, a plant utilized in folklore medicine. Bilobalide, a sesquiterpene trilactone, exhibits excellent antioxidant activity. But the molecular mechanisms associated with such effects, especially the antioxidant-related mechanism, have not been documented. Hence, this investigation explored whether bilobalide may attenuate DR in streptozotocin (STZ)-prompted diabetic rats. The effects of bilobalide on parameters of antioxidant content, oxidative stress, and inflammatory factors in the retinal tissues were evaluated by ELISA, RT-PCR, and immunohistochemistry methods. Bilobalide improved caloric management by reducing food consumption and increasing body weight. Furthermore, the administration of bilobalide decreases the blood glucose level and glycosylated (HbA1c) hemoglobin. The anti-retinopathy activity of bilobalide was established by the increase in the total retina thickness (TRT), inner nuclear layer (INL), and outer nuclear layer (ONL) in diabetic rats. Additionally, the serum level of MDA was decreased. In contrast, the antioxidant enzyme (SOD and CAT) levels were increased with TAC plus lower Keap1 and higher Nrf2 expression in the retina when associated with the DM rats. Moreover, bilobalide increased the nuclear factor erythroid 2-related factor 2 (Nrf2) and Heme oxygenase-1 (HO-1) expression level and inflammatory mediators (NF-κβ p65, TNF-α, IL-1β, and VEGF), thus inhibiting oxidative stress. Bilobalide can be effective against DR, and the possible mechanism may be relatively elucidated by decreasing oxidative stress and anti-inflammatory activities. But the further investigation should be directed to expose the precise mechanism.







Similar content being viewed by others
Data Availability
Not applicable.
References
Shinoda, K., Rejdak, R., Schuettauf, F., Blatsios, G., Völker, M., Tanimoto, N., et al. (2007). Early electroretinographic features of streptozotocin-induced diabetic retinopathy. 35(9), 847–54.
Nagai, N., Deguchi, S., Otake, H., Hiramatsu, N., Yamamoto NJIjoms. (2017). Therapeutic effect of cilostazol ophthalmic nanodispersions on retinal dysfunction in streptozotocin-induced diabetic rats. International Journal of Molecular Sciences,18(9),1971.
Stewart, MW., (2017). Diabetic retinopathy: Current pharmacologic treatment and emerging strategies: Springer.
Cho, N., Shaw, J., Karuranga, S., Huang, Y., da Rocha Fernandes, J., Ohlrogge, A., et al. (2018). IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. 138,271–81.
Chandrasekaran, P. R., Madanagopalan, V. G., & Narayanan, R. (2021). Diabetic retinopathy in pregnancy - A review. Indian Journal of Ophthalmology, 69(11), 3015–3025. https://doi.org/10.4103/ijo.IJO_1377_21.
Chistiakov DAJD, Research MSC. (2011). Reviews. Diabetic retinopathy: Pathogenic mechanisms and current treatments. 5(3),165-72.
Raman, R., & Sripriya, S. (2022). Gene therapy in diabetic retinopathy (pp. 187–194). Springer.
Chen, H., Qiu, B., Gao, G., Chen, Y., Min, H., Wu, ZJEER. (2022). Proteomic changes of aqueous humor in proliferative diabetic retinopathy patients treated with different intravitreal anti-VEGF agents. 216:108942.
Sarkar, T., Salauddin, M., Roy, A., Sharma, N., Sharma, A., Yadav, S., et al. (2022). Minor tropical fruits as a potential source of bioactive and functional foods. Critical Reviews in Food Science and Nutrition. 1–45. https://doi.org/10.1080/10408398.2022.2033953.
Sarkar, T., Salauddin, M., & Chakraborty, R. (2020). In-depth pharmacological and nutritional properties of bael (Aegle marmelos): A critical review. Journal of Agriculture and Food Research, 2, 100081. https://doi.org/10.1016/j.jafr.2020.100081.
Julius, A., Renuka, RR., Hopper, W., Babu Raghu, P., Rajendran, S., Srinivasan, S., et al. (2022). Inhibition of aldose reductase by novel phytocompounds: A heuristic approach to treating diabetic retinopathy. Evidence-Based Complementary and Alternative Medicine, 2022, 9624118.
Shi, X., Liao, S., Mi, H., Guo, C., Qi, D., Li, F., et al. (2012). Hesperidin prevents retinal and plasma abnormalities in streptozotocin-induced diabetic rats. 17(11), 12868–81.
Defeudis FVJPR. (2002). Bilobalide and neuroprotection. 46(6),565–8.
Ma, T., Lv, L., Yu, Y., Jia, L., Song, X., Xu, X., et al. (2022). Bilobalide exerts anti-inflammatory effects on chondrocytes through the AMPK/SIRT1/mTOR pathway to attenuate ACLT-induced post-traumatic osteoarthritis in rats. Frontiers in Pharmacology, 213, 783506.
Lu, J., Xie, L., Liu, K., Zhang, X., Wang, X., Dai, X., et al. (2021). Bilobalide: A review of its pharmacology, pharmacokinetics, toxicity, and safety. 35(11),6114–30.
Jaganjac, M., Milkovic, L., Zarkovic, N., & Zarkovic, K. J. F. R. B. (2022). Medicine. Oxidative Stress and Regeneration, 181, 154–165.
Yu, H., Dong, L.-H., Zhang, Y., & Liu, Q. (2022). A network pharmacology-based strategy for predicting the protective mechanism of Ginkgo biloba on damaged retinal ganglion cells. Chinese Journal of Natural Medicines, 20(1), 54–66. https://doi.org/10.1016/S1875-5364(21)60109-7.
Cheng, D., Liang, B., & Li, Y. (2013). Antihyperglycemic effect of Ginkgo biloba extract in streptozotocin-induced diabetes in rats. BioMed Research International, 2013, 162724. https://doi.org/10.1155/2013/162724.
Zhao, M., Qin, J., Shen, W., & Wu, A. (2021). Bilobalide enhances AMPK Activity to improve liver injury and metabolic disorders in STZ-induced diabetes in immature rats via regulating HMGB1/TLR4/NF-kB signaling pathway. BioMed Research International, 2021, 8835408. https://doi.org/10.1155/2021/8835408.
Khalil, ASM., Giribabu, N., Yelumalai, S., Shahzad, H., Kilari, EK., Salleh, NJLs., (2021). Myristic acid defends against testicular oxidative stress, inflammation, apoptosis: Restoration of spermatogenesis, steroidogenesis in diabetic rats. 278,119605.
Hupy, ML., Pedler, MG., Shieh, B., Wang, D., Wang, X-J., Petrash, JMJC-bi. (2021). Suppression of epithelial to mesenchymal transition markers in mouse lens by a Smad7-based recombinant protein. 344,109495.
Nayak, S. S., & Pattabiraman, T. N. (1981). A new colorimetric method for the estimation of glycosylated hemoglobin. Clinica Chimica acta International Journal of Clinical Chemistry, 109(3), 267–74. https://doi.org/10.1016/0009-8981(81)90312-0.
Ogurtsova, K., Guariguata, L., Barengo, NC., Ruiz, PL-D., Sacre, JW., Karuranga, S., et al. (2022). IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. 183,109118.
Lewis, GF., Carpentier, A., Adeli, K., Giacca AJEr. (2002). Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. 23(2):201–29.
Ostri, C., la Cour, M., Lund-Andersen, HJAo. (2014). Diabetic vitrectomy in a large type 1 diabetes patient population: Long-term incidence and risk factors. 92(5):439–43.
Circu, ML., Aw, TYJFRB. (2010). Medicine. Reactive oxygen species, cellular redox systems, and apoptosis. 48(6),762-62.
Gao, Q., Shen, W., Qin, W., Zheng, C., Zhang, M., Zeng, C., et al. (2010). Treatment of db/db diabetic mice with triptolide: A novel therapy for diabetic nephropathy. 25(11),3539-47.
Karunakaran, U., Park, K-GJD. (2013). journal m. A systematic review of oxidative stress and safety of antioxidants in diabetes: Focus on islets and their defense. 37(2),106-12.
Sahel, J.-A., & Marazova, K. (2015). Audo IJCSHpim. Clinical Characteristics and Current Therapies for Inherited Retinal Degenerations, 5(2), a017111.
Ryals, RC., Andrews, MD., Datta, S., Coyner, AS., Fischer, CM., Wen, Y., et al. (2017). Long-term characterization of retinal degeneration in Royal College of Surgeons rats using spectral-domain optical coherence tomography. 58(3),1378-86.
Carpi-Santos, R., de Melo Reis, RA., Gomes, FCA., Calaza, KCJA. (2022). Contribution of Müller cells in the diabetic retinopathy development: Focus on oxidative stress and inflammation. 11(4),617.
Ye, C-L., Hu, W-L., Dai, D-HJIjobm. (2011). Extraction of polysaccharides and the antioxidant activity from the seeds of Plantagoasiatica L. 49(4),466-70.
Bucolo, C., & Drago, F. J. P. (2011). therapeutics. Carbon Monoxide and the Eye: Implications for Glaucoma Therapy, 130(2), 191–201.
Lou, J., Cao, G., Li, R., Liu, J., Dong, Z., Xu, LJNr. (2016). β-caryophyllene attenuates focal cerebral ischemia-reperfusion injury by Nrf2/HO-1 pathway in rats. 41(6),1291–304.
He, M., Pan, H., Chang, RC-C., So, K-F., Brecha, NC., Pu, MJPo. (2014). Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage. 9(1),e84800.
Takeda, K., Kermani, P., Anastasia, A., Obinata, Y., Hempstead, BL., Kurihara, HJB., et al. (2013). BDNF protects human vascular endothelial cells from TNFα-induced apoptosis. 91(5),341-349.
Gao, J-J., Hu, Y-W., Wang, Y-C., Sha, Y-H., Ma, X., Li, S-F., et al. (2015). ApoM suppresses TNF-α-induced expression of ICAM-1 and VCAM-1 through inhibiting the activity of NF-κB. 34(8),550–6.
Author information
Authors and Affiliations
Contributions
All authors contributed equally.
Corresponding author
Ethics declarations
Consent to Participate
All authors have their consent to participate.
Consent to Publish
All authors have their consent to publish their work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Su, Q., Dong, J., Zhang, D. et al. Protective Effects of the Bilobalide on Retinal Oxidative Stress and Inflammation in Streptozotocin-Induced Diabetic Rats. Appl Biochem Biotechnol 194, 6407–6422 (2022). https://doi.org/10.1007/s12010-022-04012-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04012-5