Abstract
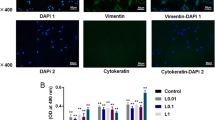
Osteoporosis is a general bone-related ailment characterized by reduced bone density and quality, elevated bone fragility, and fractures. It was reported that both aged men and women has an increased risks of osteoporosis. The current research work focused to unveil the beneficial roles of ponicidin treatment in the proliferation and calcium deposition on the osteoblast-like MG-63 cells. The effect of 5 and 10 µg/ml of ponicidin on the cell proliferation was assessed. The viability of ponicidin-supplemented MG-63 cells was inspected by MTT test. The contents of osteocalcin, collagen, and ALP activity in the ponicidin administered cells were assessed by kits. The level of calcium mineralization was examined by ARS staining technique. The ponicidin treatment remarkably improved the proliferation of MG-63 cells. The ponicidin did not affect the MG-63 cells viability but promoted its viability 24- and 48-h treatment. The contents of osteocalcin, collagen, and ALP activity in the 5 and 10 µg/ml of ponicidin-supplemented MG-63 cells were found increased than the control cells. The ponicidin also increased the level of calcium deposition in MG-63 cells, which is assessed by ARS staining. In conclusion, it was clear that ponicidin improved the proliferation and calcium mineralization in a MG-63 cells. Therefore, it was clear that ponicidin has helpful roles on the new bone development as a hopeful therapeutic candidate to treat the bone-related disease like osteoporosis.




Similar content being viewed by others
Data Availability
Not applicable.
References
Curate, F. (2014). Osteoporosis and paleopathology: A review. Journal of Anthropological Sciences, 92, 119–146.
Zhao, X., Wu, Z. X., Zhang, Y., Gao, M. X., Yan, Y. B., Cao, P. C., Zang, Y., & Lei, W. (2012). Locally administrated perindopril improves healing in an ovariectomized rat tibial osteotomy model. PLoS One, 7, e33228.
Kling, J. M., Clarke, B. L., & Sandhu, N. P. (2014). Osteoporosis prevention, screening, and treatment: A review. Journal of Women’s Health, 23, 563–572.
Zhang, S., Wu, W., Jiao, G., Li, C., & Liu, H. (2018). MiR-455-3p activates Nrf2/ARE signaling via HDAC2 and protects osteoblasts from oxidative stress. International Journal of Biological Macromolecules, 107, 2094–2101.
Cauley, J. A., Chalhoub, D., Kassem, A. M., & Fuleihan, G. E. H. (2014). Geographic and ethnic disparities in osteoporotic fractures. Nature Reviews. Endocrinology, 10, 338.
Rao, L., & Rao, A. (2015). Oxidative stress and antioxidants in the risk of osteoporosis — Role of phytochemical antioxidants lycopene and polyphenol-containing nutritional supplements. In A. V. Rao & L. G. Rao (Eds.), Phytochemicals - Isolation, characterisation and role in human health [Internet]. IntechOpen [cited 2022 May 11]. Available from: https://www.intechopen.com/chapters/49068, https://doi.org/10.5772/60446.
Tsao, Y. T., Huang, Y. J., Wu, H. H., Liu, Y. A., Liu, Y. S., & Lee, O. K. (2017). Osteocalcin mediates biomineralization during osteogenic maturation in human mesenchymal stromal Cells. International Journal of Molecular Sciences, 18, 159.
Klumpers, D. D., Zhao, X., Mooney, D. J., & Smit, T. H. (2013). Cell mediated contraction in 3D cell-matrix constructs leads to spatially regulated osteogenic differentiation. Integr Biol (Camb), 5, 1174–1183.
Liu, T. M., & Lee, E. H. (2013). Transcriptional regulatory cascades in Runx2-dependent bone development. Tissue Engineering. Part B, Reviews, 19(3), 254–263.
Cheung, A. S., Pattison, D., Bretherton, I., Hoermann, R., Lim, J. D., Ho, E., … Grossmann, M. (2013). Cardiovascular risk and bone loss in men undergoing androgen deprivation therapy for non-metastatic prostate cancer: Implementation of standardized management guidelines. Andrology, 1, 583-589.
Gambacciani, M., & Levancini, M. (2014). Management of postmenopausal osteoporosis and the prevention of fractures. Panminerva Medica, 56, 115–131.
Park, H. S., Kim, C. G., Hong, N., Lee, S. J., Seo, D. H., & Rhee, Y. (2017). Osteosarcoma in a patient with pseudo-hypoparathyroidism type 1b due to paternal uniparental disomy of chromosome 20q. Journal of Bone and Mineral Research, 32, 770–775.
Bhutani, G., & Gupta, M. C. (2013). Emerging therapies for the treatment of osteoporosis. J Midlife Health, 4, 147–152.
Li, C., Li, Q., Liu, R., Niu, Y., Pan, Y., Zhai, Y., & Mei, Q. (2014). Medicinal herbs in the prevention and treatment of osteoporosis. American Journal of Chinese Medicine, 42, 1–22.
Zhao, W., Pu, J. X., Du, X., Su, J., Li, X. N., Yang, J. H., & Sun, H. D. (2011). Structure and cytotoxicity of diterpenoids from Isodon adenolomus. Journal of Natural Products, 74, 1213–1220.
Osawa, K., Yasuda, H., Maruyama, T., Morita, H., Takeya, K., & Itokawa, H. (1994). Antibacterial trichorabdal diterpenes from Rabdosia trichocarpa. Phytochemistry, 36, 1287–1291.
Zhang, J. F., Liu, P. Q., Chen, G. H., Lu, M. Q., Cai, C. J., Yang, Y., & Li, H. (2007). Ponicidin inhibits cell growth on hepatocellular carcinoma cells by induction of apoptosis. Digestive and Liver Disease, 39, 160–166.
Liu, J. J., Huang, R. W., Lin, D. J., Peng, J., Zhang, M., Pan, X., … Chen, F. (2006). Ponicidin, an ent-kaurane diterpenoid derived from a constituent of the herbal supplement PC-SPES, Rabdosia rubescens, induces apoptosis by activation of caspase-3 and mitochondrial events in lung cancer cells in vitro. Cancer Invest, 24, 136-148.
Bai, N., He, K., Zhou, Z., Tsai, M. L., Zhang, L., Quan, Z., … Ho, C. T. Ent-kaurane diterpenoids from Rabdosia rubescens and their cytotoxic effects on human cancer cell lines. Planta Med ,76, 140–145.
Liu, J. J., Zhang, Y., Guang, W. B., Yang, H. Z., Lin, D. J., & Xiao, R. Z. (2008). Ponicidin inhibits monocytic leukemia cell growth by induction of apoptosis. International Journal of Molecular Sciences, 9, 2265–2277.
Hsieh, T. C., Wijeratne, E. K., Liang, J. Y., Gunatilaka, A. L., & Wu, J. M. (2005). Differential control of growth, cell cycle progression, and expression of NF-kappaB in human breast cancer cells MCF-7, MCF-10A, and MDA-MB-231 by ponicidin and oridonin, diterpenoids from the Chinese herb Rabdosia rubescens. Biochemical and Biophysical Research Communications, 337, 224–231.
Liu, Y. F., Lu, Y. M., Qu, G. Q., Liu, Y., Chen, W. X., Liao, X. H., & Kong, W. M. (2015). Ponicidin induces apoptosis via JAK2 and STAT3 signaling pathways in gastric carcinoma. International Journal of Molecular Sciences, 16, 1576–1589.
Du, J., Chen, C., Sun, Y., Zheng, L., & Wang, W. (2015). Ponicidin suppresses HT29 cell growth via the induction of G1 cell cycle arrest and apoptosis. Molecular Medicine Reports, 12, 5816–5820.
Armas, L. A. (2012). Recker RR Pathophysiology of osteoporosis: New mechanistic insights. Endocrinology and Metabolism Clinics of North America, 41(3), 475–486.
Burge, R., Dawson-Hughes, B., Solomon, D. H., Wong, J. B., & King, A. (2007). Tosteson A Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. Journal of Bone and Mineral Research, 22(3), 465–475.
Reid, I. R. (2015). Short-term and long-term effects of osteoporosis therapies. Nature Reviews. Endocrinology, 11(7), 418–428.
Coughlan, T., & Dockery, F. (2014). Osteoporosis and fracture risk in older people. Clinical Medicine, 14(2), 187–191.
Brown, C. (2017). Osteoporosis: Staying strong. Nature, 550(7674), S15–S17.
Chen, H., Senda, T., & Kubo, K. Y. (2015). The osteocyte plays multiple roles in bone remodeling and mineral homeostasis. Medical Molecular Morphology, 48(2), 61–68.
Valiya Kambrath, A., Williams, J. N., & Sankar, U. (2020). An improved methodology to evaluate cell and molecular signals in the reparative callus during fracture healing. Journal of Histochemistry and Cytochemistry, 2020(68), 199–208.
Bonjour, J. P., Chevalley, T., Ferrari, S., & Rizzoli, R. (2009). The importance and relevance of peak bone mass in the prevalence of osteoporosis. Salud Publica de Mexico, 51(Suppl 1), S5–S17.
DiMasi, J. A., Hansen, R. W., & Grabowski, H. G. (2003). The price of innovation: New estimates of drug development costs. Journal of Health Economics, 22, 151–185.
Wegler, C., Wikvall, K., & Norlin, M. (2016). Effects of osteoporosis-inducing drugs on vitamin d-related gene transcription and mineralization in MG-63 and Saos-2 Cells. Basic & Clinical Pharmacology & Toxicology, 119(5), 436–442.
Zofkova, I., Davis, M., & Blahos, J. (2017). Trace elements have beneficial, as well as detrimental effects on bone homeostasis. Physiological Research, 66(3), 391–402.
Plikerd, W. D., Trivedi, M. K., Branton, A., Trivedi, D., Nayak, G., Gangwar, M., & Jana, S. (2018). Impact of biofield energy healing treated Vitamin D3 on human osteoblast cell line (MG-63) for bone health. American Journal of Clinical and Experimental Medicine, 6(1), 1–9.
Raggatt, L. J., & Partridge, N. C. (2010). Cellular and molecular mechanisms of bone remodeling. Journal of Biological Chemistry, 285, 25103–25108.
Zaidi, M. (2007). Skeletal remodeling in health and disease. Nature Medicine, 13, 791–801.
Twine, N. A., Chen, L., Pang, C. N., Wilkins, M. R., & Kassem, M. (2014). Identification of differentiation-stage specific markers that define the ex vivo osteoblastic phenotype. Bone, 67, 23–32.
Sudo, H., Kodama, H. A., Amagai, Y., Yamamoto, S., & Kasai, S. (1983). In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. Journal of Cell Biology, 96, 191–198.
Ducy, P., Schinke, T., & Karsenty, G. (2001). The osteoblast: A sophisticated fibroblast under central surveillance. Science, 289, 1501–1504.
Kostenuik, P. J., Halloran, B. P., Morey-Holton, E. R., & Bikle, D. D. (1997). Skeletal unloading inhibits the in vitro proliferation and differentiation of rat osteoprogenitor cells. American Journal of Physiology, 273, E1133–E1139.
Kostenuik, P. J., Harris, J., Halloran, B. P., Turner, R. T., Morey-Holton, E. R., & Bikle, D. D. (1999). Skeletal unloading causes resistance of osteoprogenitor cells to parathyroid hormone and to insulin-like growth factor-I. J Bone Min Res, 14, 21–31.
Kalajzic, I., Staal, A., Yang, W. P., Wu, Y., Johnson, S. E., Feyen, J. H., … Rowe, D. W. (2005). Expression profile of osteoblast lineage at defined stages of differentiation. J Biol Chem, 280, 24618-24626.
Kotliarova, M. S., Zhuikov, V. A., Chudinova, Y. V., Khaidapova, D. D., Moisenovich, A. M., & Kon’kov, A. S., … Shaitan, K. V. (2016). Induction of osteogenic differentiation of osteoblast-like cells MG-63 during cultivation on fibroin microcarriers. Moscow University Biological Science Bulletin, 71(4), 212–217.
Chaturvedi, R., Singha, P. K., & Dey, S. (2013). Water soluble bioactives of nacre mediate antioxidant activity and osteoblast differentiation. PLoS One, 8, e84584.
Wei, J., & Karsenty, G. (2015). An overview of the metabolic functions of osteocalcin. Reviews in Endocrine & Metabolic Disorders, 16, 93.
Marie, P. J. (2002). Role of N-cadherin in bone formation. Journal of Cellular Physiology, 190, 297–305.
Feng, H., Cheng, T., Pavlos, N. J., Yip, K. H., Carrello, A., Seeber, R., Eidne, K., Zheng, M. H., & Xu, J. (2008). Cytoplasmic terminus of vacuolar type proton pump accessory subunit Ac45 is required for proper interaction with V(0) domain subunits and efficient osteoclastic bone resorption. Journal of Biological Chemistry, 283(19), 13194–13204.
Feng, J., Shi, Z., & Ye, Z. (2008). Effects of metabolites of the lignans enterolactone and enterodiol on osteoblastic differentiation of MG-63 cells. Biological &/and Pharmaceutical Bulletin, 31(6), 1067–1070.
Kasperk, C., Wergedal, J., Strong, D., Farley, J., Wangerin, K., Gropp, H., Ziegler, R., & Baylink, D. J. (1995). Human bone cell phenotypes differ depending on their skeletal site of origin. Journal of Clinical Endocrinology and Metabolism, 80, 2511–2517.
Pochampally, R. R., Horwitz, E. M., DiGirolamo, C. M., Stokes, D. S., & Prockop, D. J. (2005). Correction of a mineralization defect by overexpression of a wild-type cDNA for COL1A1 in marrow stromal cells (MSCs) from a patient with osteogenesis imperfecta: A strategy for rescuing mutations that produce dominant-negative protein defects. Gene Therapy, 12(14), 1119–1125.
Sakkers, R., Kok, D., Engelbert, R., van Dongen, A., Jansen, M., Pruijs, H., … Uiterwaal, C. (2004). Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: A 2-year randomised placebo-controlled study. Lancet, 363(9419), 1427–1431.
Mathews, S., Gupta, P. K., Bhonde, R., & Totey, S. (2011). Chitosan enhances mineralization during osteoblast differentiation of human bone marrow-derived mesenchymal stem cells, by upregulating the associated genes. Cell Proliferation, 44(6), 537–549.
Reffitt, D. M., Ogston, N., Jugdaohsingh, R., Cheung, H. F., Evans, B. A., Thompson, R. P., Powell, J. J., & Hampson, G. N. (2003). Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone, 32, 127–135.
Wen, L. P., Chen, J. P., Duan, L. L., & Li, S. Z. (2018). Vitamin K-dependent proteins involved in bone and cardiovascular health. Molecular Medicine Reports, 18, 3–15.
Cosman, F., Shen, V., Morgan, D., Gordon, S., Parisien, M., Nieves, J., & Lindsay, R. (2000). Biochemical responses of bone metabolism to 1,25-dihydroxyvitamin D administration in black and white women. Osteoporosis International, 11(3), 271–277.
Mizokami, A., Kawakubo-Yasukochi, T., & Hirata, M. (2017). Osteocalcin and its endocrine functions. Biochemical Pharmacology, 132, 1–8.
Diegel, C. R., Hann, S., Ayturk, U. M., Hu, J. C. W., Lim, K. E., Droscha, C. J., … Williams, B. O. (2020). An osteocalcin-deficient mouse strain without endocrine abnormalities. PLoS Genet, 16, 1008361.
Moriishi, T., Ozasa, R., Ishimoto, T., Nakano T., Hasegawa T., Miyazaki T., … Komori, T. (2020). Osteocalcin is necessary for the alignment of apatite crystallites, but not glucose metabolism, testosterone synthesis, or muscle mass. PLoS Genet, 16, e1008586.
Author information
Authors and Affiliations
Contributions
All authors contributed equally.
Corresponding author
Ethics declarations
Ethics Approval
All works have been done under the guidelines of Institutional Ethics Committee.
Consent to Participate
All authors have their consent to participate.
Consent for Publication
All authors have their consent to publish their work.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Hao, P., Li, H. et al. Ponicidin Treatment Improved the Cell Proliferation, Differentiation, and Calcium Mineralization on the Osteoblast-Like MG-63 Cells. Appl Biochem Biotechnol 194, 3860–3870 (2022). https://doi.org/10.1007/s12010-022-03927-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03927-3