Abstract
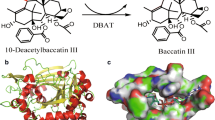
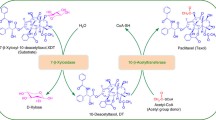
Taxoid 10β-O-acetyl transferase (DBAT) is a key enzyme in the biosynthesis of the famous anticancer drug paclitaxel, which catalyses the formation of baccatin III from 10-deacetylbaccatin III (10-DAB). However, the activity essential residues of the enzyme are still unknown, and the acylation mechanism from its natural substrate 10-deacetylbaccatin III and acetyl CoA to baccatin III remains unclear. In this study, the homology modelling, molecular docking, site-directed mutagenesis, and kinetic parameter determination of the enzyme were carried out. The results showed that the enzyme mutant DBATH162A resulted in complete loss of enzymatic activity, suggesting that the residue histidine at 162 was essential to DBAT activity. Residues D166 and R363 which were located in the pocket of the enzyme by homology modelling and molecular docking were also important for DBAT activity through the site-directed mutations. Furthermore, four amino acid residues including S31 and D34 from motif SXXD, D372 and G376 from motif DFGWG also played important roles on acylation. This was the first report of the elucidation of the activity essential residues of DBAT, making it possible for the further structural-based re-design of the enzyme for efficient biotransformation of baccatin III and paclitaxel.




Similar content being viewed by others
References
Jibodh, R. A., Lagas, J. S., Nuijen, B., Beijnen, J. H., & Schellens, J. H. (2013). Taxanes: old drugs, new oral formulations. European Journal of Pharmacology, 717(1-3), 40–46.
Nobili, S., Lippi, D., Witort, E., Donnini, M., Bausi, L., Mini, E., & Capaccioli, S. (2009). Natural compounds for cancer treatment and prevention. Pharmacological Research, 59(6), 365–378.
Wang, Y. F., Shi, Q. W., Dong, M., Kiyota, H., Gu, Y. C., & Cong, B. (2011). Natural taxanes: developments since 1828. Chemical Reviews, 111(12), 7652–7709.
Mukherjee, S., Ghosh, B., Jha, T. B., & Jha, S. (2002). Variation in content of taxol and related taxanes in eastern Himalayan populations of Taxus wallichiana. Planta Medica, 68(8), 757–759.
Han, F., Kang, L. Z., Zeng, X. L., Ye, Z. W., Guo, L. Q., & Lin, J. F. (2014). Bioproduction of baccatin III, an advanced precursor of paclitaxol, with transgenic Flammulina velutipes expressing the 10-deacetylbaccatin III-10-O-acetyl transferase gene. Journal of the Science of Food and Agriculture, 94(12), 2376–2383.
Yang, L., Yang, C., Li, C., Zhao, Q., Liu, L., Fang, X., & Chen, X. Y. (2016). Recent advances in biosynthesis of bioactive compounds in traditional Chinese medicinal plants. Scientific Bulletin (Beijing), 61(1), 3–17.
Croteau, R., Ketchum, R. E., Long, R. M., Kaspera, R., & Wildung, M. R. (2006). Taxol biosynthesis and molecular genetics. Phytochemistry Reviews, 5(1), 75–97.
Guerra-Bubb, J., Croteau, R., & Williams, R. M. (2012). The early stages of taxol biosynthesis: an interim report on the synthesis and identification of early pathway metabolites. Natural Product Reports, 29(6), 683–696.
Cheynier, V., Comte, G., Davies, K. M., Lattanzio, V., & Martens, S. (2013). Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiology and Biochemistry, 72, 1–20.
Dang, T. T., Chen, X., & Facchini, P. J. (2015). Acetylation serves as a protective group in noscapine biosynthesis in opium poppy. Nature Chemical Biology, 11(2), 104–106.
Walker, K., & Croteau, R. (2000). Molecular cloning of a 10-deacetylbaccatin III-10-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America, 97(2), 583–587.
Loncaric, C., Merriweather, E., & Walker, K. D. (2006). Profiling a taxol pathway 10beta-acetyltransferase: assessment of the specificity and the production of baccatin III by in vivo acetylation in E. coli. Chemistry & Biology, 13(3), 309–317.
Chau, M., Walker, K., Long, R., & Croteau, R. (2004). Regioselectivity of taxoid-O-acetyltransferases: heterologous expression and characterization of a new taxadien-5alpha-ol-O-acetyltransferase. Archives of Biochemistry and Biophysics, 430(2), 237–246.
Hai, P., Wen, S. Z., Li, Y., Gao, Y., Jiang, X. J., & Wang, F. (2014). New taxane diterpenoids from Taxus yunnanensis. Natural Products and Bioprospecting, 4(1), 47–51.
Ondari, M. E., & Walker, K. D. (2008). The taxol pathway 10-O-acetyltransferase shows regioselective promiscuity with the oxetane hydroxyl of 4-deacetyltaxanes. Journal of the American Chemical Society, 130(50), 17187–17194.
Loncaric, C., Ward, A. F., & Walker, K. D. (2007). Expression of an acetyl-CoA synthase and a CoA-transferase in Escherichia coli to produce modified taxanes in vivo. Biotechnology Journal, 2(2), 266–274.
Koksal, M., Jin, Y., Coates, R. M., Croteau, R., & Christianson, D. W. (2011). Taxadiene synthase structure and evolution of modular architecture in terpene biosynthesis. Nature, 469(7328), 116–120.
Wildung, M. R., & Croteau, R. (1996). A cDNA clone for Taxadiene synthase, the Diterpene cyclase that catalyzes the committed step of Taxol biosynthesis. The Journal of Biological Chemistry, 271(16), 9201–9204.
Williams, D. C., Carroll, B. J., Jin, Q., Rithner, C. D., Lenger, S. R., Floss, H. G., Coates, R. M., Williams, R. M., & Croteau, R. (2000). Intramolecular proton transfer in the cyclization of geranylgeranyl diphosphate to the taxadiene precursor of taxol catalyzed by recombinant taxadiene synthase. Chemistry & Biology, 7(12), 969–977.
Bontpart, T., Cheynier, V., Ageorges, A., & Terrier, N. (2015). BAHD or SCPL acyltransferase? What a dilemma for acylation in the world of plant phenolic compounds. The New Phytologist, 208(3), 695–707.
Lallemand, L. A., Zubieta, C., Lee, S. G., Wang, Y., Acajjaoui, S., Timmins, J., McSweeney, S., Jez, J. M., McCarthy, J. G., & McCarthy, A. A. (2012). A structural basis for the biosynthesis of the major chlorogenic acids found in coffee. Plant Physiology, 160(1), 249–260.
Walker, A. M., Hayes, R. P., Youn, B., Vermerris, W., Sattler, S. E., & Kang, C. (2013). Elucidation of the structure and reaction mechanism of sorghum hydroxycinnamoyltransferase and its structural relationship to other coenzyme a-dependent transferases and synthases. Plant Physiology, 162(2), 640–651.
John, B., & Sali, A. (2003). Comparative protein structure modeling by iterative alignment, model building and model assessment. Nucleic Acids Research, 31(14), 3982–3992.
Lovell, S. C., Davis, I. W., Arendall 3rd, W. B., de Bakker, P. I., Word, J. M., Prisant, M. G., Richardson, J. S., & Richardson, D. C. (2003). Structure validation by Calpha geometry: Phi,psi and Cbeta deviation. Proteins, 50(3), 437–450.
Zheng, L., Baumann, U., & Reymond, J. L. (2004). An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Research, 32(14), e115.
Takahashi, K., Hirose, Y., Kamimura, N., Hishiyama, S., Hara, H., Araki, T., Kasai, D., Kajita, S., Katayama, Y., Fukuda, M., & Masai, E. (2015). Membrane-associated glucose-methanol-choline oxidoreductase family enzymes PhcC and PhcD are essential for enantioselective catabolism of dehydrodiconiferyl alcohol. Applied and Environmental Microbiology, 81(23), 8022–8036.
Winzer, T., Kern, M., King, A. J., Larson, T. R., Teodor, R. I., Donninger, S. L., Li, Y., Dowle, A. A., Cartwright, J., Bates, R., Ashford, D., Thomas, J., Walker, C., Bowser, T. A., & Graham, I. A. (2015). Plant science. Morphinan biosynthesis in opium poppy requires a P450-oxidoreductase fusion protein. Science, 349(6245), 309–312.
Morales-Quintana, L., Fuentes, L., Gaete-Eastman, C., Herrera, R., & Moya-Leon, M. A. (2011). Structural characterization and substrate specificity of VpAAT1 protein related to ester biosynthesis in mountain papaya fruit. Journal of Molecular Graphics & Modelling, 29(5), 635–642.
Unno, H., Ichimaida, F., Suzuki, H., Takahashi, S., Tanaka, Y., Saito, A., Nishino, T., Kusunoki, M., & Nakayama, T. (2007). Structural and mutational studies of anthocyanin malonyltransferases establish the features of BAHD enzyme catalysis. The Journal of Biological Chemistry, 282(21), 15812–15822.
Manjasetty, B. A., Yu, X. H., Panjikar, S., Taguchi, G., Chance, M. R., & Liu, C. J. (2012). Structural basis for modification of flavonol and naphthol glucoconjugates by Nicotiana tabacum malonyltransferase (NtMaT1). Planta, 236(3), 781–793.
Molina, I., & Kosma, D. (2014). Role of HXXXD-motif/BAHD acyltransferases in the biosynthesis of extracellular lipids. Plant Cell Reports, 34, 587–601.
Ma, X., Koepke, J., Panjikar, S., Fritzsch, G., & Stockigt, J. (2005). Crystal structure of vinorine synthase, the first representative of the BAHD superfamily. The Journal of Biological Chemistry, 280(14), 13576–13583.
Li, B. J., Wang, H., Gong, T., Chen, J. J., Chen, T. J., Yang, J. L., & Zhu, P. (2017). Improving 10-deacetylbaccatin III-10-beta-O-acetyltransferase catalytic fitness for Taxol production. Nature Communications, 8, 15544.
Galaz, S., Morales-Quintana, L., Moya-Leon, M. A., & Herrera, R. (2013). Structural analysis of the alcohol acyltransferase protein family from Cucumis melo shows that enzyme activity depends on an essential solvent channel. The FEBS Journal, 280(5), 1344–1357.
Bayer, A., Ma, X., & Stockigt, J. (2004). Acetyltransfer in natural product biosynthesis--functional cloning and molecular analysis of vinorine synthase. Bioorganic & Medicinal Chemistry, 12(10), 2787–2795.
Gerasimenko, I., Ma, X., Sheludko, Y., Mentele, R., Lottspeich, F., & Stockigt, J. (2004). Purification and partial amino acid sequences of the enzyme vinorine synthase involved in a crucial step of ajmaline biosynthesis. Bioorganic & Medicinal Chemistry, 12(10), 2781–2786.
Tuominen, L. K., Johnson, V. E., & Tsai, C. J. (2011). Differential phylogenetic expansions in BAHD acyltransferases across five angiosperm taxa and evidence of divergent expression among Populus paralogues. BMC Genomics, 12(1), 236.
Acknowledgements
This work was supported by the Science and Technology Program of Guangdong Province (grant number 2014B050505018, 2014B020205003, 2015A020209121) and the National Natural Science Foundation of China (grant number 31071837, 31372116, 31572178).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interests.
Electronic Supplementary Material
ESM 1
(DOC 659 kb)
Rights and permissions
About this article
Cite this article
You, LF., Wei, T., Zheng, QW. et al. Activity Essential Residue Analysis of Taxoid 10β-O-Acetyl Transferase for Enzymatic Synthesis of Baccatin. Appl Biochem Biotechnol 186, 949–959 (2018). https://doi.org/10.1007/s12010-018-2789-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2789-0