Abstract
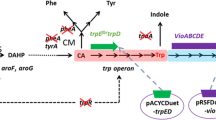
Crude violacein, consisting of violacein and deoxyviolacein, displays many attractive bio-activities in the field of drug therapy. To produce crude violacein from an industrially economic carbon source, we firstly introduced the violacein pathway into Escherichia coli B8/pTRPH1, which was previously engineered to accumulate tryptophan from glucose. A crude violacein production capacity of 0.25 g L−1 OD600−1 was obtained using glucose-containing medium. By further overexpressing each of the five genes involved in violacein synthesis pathway, VioE was found as the rate-limiting step for the violacein production. The optimal strain of B8/pTRPH1-pVio-VioE was then used for fed-batch fermentation in a 5-L bioreactor and a crude violacein titer of 4.45 g L−1, as well as a productivity of 98.7 mg L−1 h−1, was obtained. This engineered strain showed the highest violacein titer and productivity reported so far. Our optimal strain of E. coli B8/pTRPH1-pVio-VioE by overexpression of the rate-limiting VioE in violacein synthesis pathway was a potential violacein producer by directly using glucose for industrial application.



Similar content being viewed by others
References
Nakamura, Y., Asada, C., & Sawada, T. (2003). Production of antibacterial violet pigment by psychrotropic bacterium RT102 strain. Biotechnology and Bioprocess Engineering, 8(1), 37–40. https://doi.org/10.1007/BF02932896.
Durán, N., Justo, G. Z., Ferreira, C. V., Melo, P. S., Cordi, L., & Martins, D. (2007). Violacein: properties and biological activities. Biotechnology and Applied Biochemistry, 48(3), 127. https://doi.org/10.1042/BA20070115.
Andrighetti-Fröhner, C. R., Antonio, R. V., Creczynski-Pasa, T. B., Barardi, C. R. M., & Simões, C. M. O. (2003). Cytotoxicity and potential antiviral evaluation of violacein produced by Chromobacterium violaceum. Memórias do Instituto Oswaldo Cruz, 98(6), 843–848. https://doi.org/10.1590/S0074-02762003000600023.
Becker, M. H., Brucker, R. M., Schwantes, C. R., Harris, R. N., & Minbiole, K. P. C. (2009). The bacterially produced metabolite violacein is associated with survival of amphibians infected with a lethal fungus. Applied and Environmental Microbiology, 75(21), 6635–6638. https://doi.org/10.1128/AEM.01294-09.
Jiang, P. X., Wang, H. S., Zhang, C., Lou, K., & Xing, X. H. (2010). Reconstruction of the violacein biosynthetic pathway from Duganella sp. B2 in different heterologous hosts. Applied Microbiology and Biotechnology, 86(4), 1077–1088. https://doi.org/10.1007/s00253-009-2375-z.
Bromberg, N., & Durán, N. (2001). Violacein transformation by peroxidases and oxidases: implications on its biological properties. Journal of Molecular Catalysis B: Enzymatic, 11(4–6), 463–467. https://doi.org/10.1016/S1381-1177(00)00171-5.
Jiang, P. X., Wang, H. S., Xiao, S., Fang, M. Y., Zhang, R. P., He, S. Y., … Xing, X. H. (2012). Pathway redesign for deoxyviolacein biosynthesis in Citrobacter freundii and characterization of this pigment. Applied Microbiology and Biotechnology, 94(6), 1521–1532. doi:https://doi.org/10.1007/s00253-012-3960-0.
Yang, C., Jiang, P., Xiao, S., Zhang, C., Lou, K., & Xing, X. H. (2011). Fed-batch fermentation of recombinant Citrobacter freundii with expression of a violacein-synthesizing gene cluster for efficient violacein production from glycerol. Biochemical Engineering Journal, 57(1), 55–62. https://doi.org/10.1016/j.bej.2011.08.008.
Lee, M. E., Aswani, A., Han, A. S., Tomlin, C. J., & Dueber, J. E. (2013). Expression-level optimization of a multi-enzyme pathway in the absence of a high-throughput assay. Nucleic Acids Research, 41(22), 10668–10678. https://doi.org/10.1093/nar/gkt809.
Guo, Y., Dong, J., Zhou, T., Auxillos, J., Li, T., Zhang, W., … Dai, J. (2015). YeastFab: the design and construction of standard biological parts for metabolic engineering in Saccharomyces cerevisiae. Nucleic Acids Research, 43(13), e88. doi:https://doi.org/10.1093/nar/gkv464.
Zhou, Y., Li, G., Dong, J., Xing, X., Dai, J., & Zhang, C. (2018). MiYA, an efficient machine-learning workflow in conjunction with the YeastFab assembly strategy for combinatorial optimization of heterologous metabolic pathways in Saccharomyces cerevisiae. Metabolic Engineering, 47, 294–302. https://doi.org/10.1016/j.ymben.2018.03.020.
Pontrelli, S., Chiu, T., Lan, E. I., Chen, F. Y., Chang, P., Liao, J. C., … Liao, J. C. (2018). Escherichia coli as a host for metabolic engineering. Metabolic Engineering. doi:https://doi.org/10.1016/j.ymben.2018.04.008.
Rodrigues, A. L., Trachtmann, N., Becker, J., Lohanatha, A. F., Blotenberg, J., Bolten, C. J., Korneli, C., de Souza Lima, A. O., Porto, L. M., Sprenger, G. A., & Wittmann, C. (2013). Systems metabolic engineering of Escherichia coli for production of the antitumor drugs violacein and deoxyviolacein. Metabolic Engineering, 20, 29–41. https://doi.org/10.1016/j.ymben.2013.08.004.
Rodrigues, A. L., Becker, J., de Souza Lima, A. O., Porto, L. M., & Wittmann, C. (2014). Systems metabolic engineering of Escherichia coli for gram scale production of the antitumor drug deoxyviolacein from glycerol. Biotechnology and Bioengineering, 111(11), 2280–2289. https://doi.org/10.1002/bit.25297.
Fang, M.-Y., Zhang, C., Yang, S., Cui, J.-Y., Jiang, P.-X., Lou, K., … Xing, X.-H. (2015). High crude violacein production from glucose by Escherichia coli engineered with interactive control of tryptophan pathway and violacein biosynthetic pathway. Microbial Cell Factories, 14(1), 8. doi:https://doi.org/10.1186/s12934-015-0192-x.
Fang, M., Wang, T., Zhang, C., Bai, J., Zheng, X., Zhao, X., … Xing, X. H. (2016). Intermediate-sensor assisted push-pull strategy and its application in heterologous deoxyviolacein production in Escherichia coli. Metabolic Engineering, 33, 41–51. doi:https://doi.org/10.1016/j.ymben.2015.10.006.
Chen, Y., Xing, X. H., Ye, F., Kuang, Y., & Luo, M. (2007). Production of MBP-HepA fusion protein in recombinant Escherichia coli by optimization of culture medium. Biochemical Engineering Journal, 34(2), 114–121. https://doi.org/10.1016/j.bej.2006.11.020.
Gibson, D. G., Young, L., Chuang, R.-Y., Venter, J. C., Hutchison, C. A, Smith, H. O., … America, N. (2009). Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature Methods, 6(5), 343–5. doi:https://doi.org/10.1038/nmeth.1318.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428. https://doi.org/10.1021/ac60147a030.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC 21376137) and Tsinghua University Initiative Scientific Research Program (2013Z02-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We confirm that this manuscript has not been published elsewhere and is not under consideration by another journal. All authors have approved the manuscript and agree with submission to Applied Biochemistry and Biotechnology.
Conflict of Interest
The authors declare that they have no competing interests.
Electronic Supplementary Material
ESM 1
(DOC 47 kb)
Rights and permissions
About this article
Cite this article
Zhou, Y., Fang, MY., Li, G. et al. Enhanced Production of Crude Violacein from Glucose in Escherichia coli by Overexpression of Rate-Limiting Key Enzyme(S) Involved in Violacein Biosynthesis. Appl Biochem Biotechnol 186, 909–916 (2018). https://doi.org/10.1007/s12010-018-2787-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2787-2