Abstract
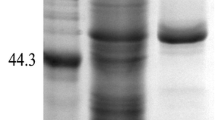
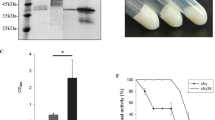
N-glycosylation usually occurs at the Asn-Xaa-Ser/Thr sequon of glycoproteins in Pichia pastoris, exerting great effects on expression efficiency; however, Asn-Xaa-Thr is more efficiently glycosylated than Asn-Xaa-Ser. In this study, the role of the two sequons in the expression of recombinant elastase (rPAE) was investigated. At N43, N212, and N280 of rPAE, Asn-Xaa-Thr was substituted for the native Asn-Xaa-Ser sequon through site-directed mutagenesis, and the two sequon forms were introduced into rPAE at N36 and N264. As expected, substitution at N36, N43, N212, and N280 enhanced the degree of N-glycosylation. At N212 or N280, substitution increased rPAE production effectively by 43 and 25 %, respectively. In comparison, at N36, N43, and N264, the change inhibited rPAE expression to varying extents; specifically, substitution at N36 resulted in a 31 % decrease, while substitution at N43 or N264 resulted in a decrease of less than 9 %. It is suggested that the effect of the substitution of Asn-Xaa-Thr for Asn-Xaa-Ser on rPAE expression is roughly related to the role of the original Asn-Xaa-Ser sequon. As the conversion of Ser to Thr at N-glycosylation sites through site-directed mutagenesis is easily achieved, it is a feasible means of improving the expression of recombinant proteins in P. pastoris.



Similar content being viewed by others
References
Bever, R. A., & Iglewski, B. H. (1988). Journal of Bacteriology, 170, 4309–4314.
Doukyu, N., & Ogino, H. (2010). Biochemical Engineering Journal, 48, 270–282.
Tang, X. Y., Wu, B., Ying, H. J., & He, B. F. (2010). Applied Biochemistry and Biotechnology, 160, 1017–1031.
Han, M. H., Ding, H. Y., Wang, J. L., Jin, M. Y., & Yu, X. B. (2013). Biochemical Engineering Journal, 77, 154–160.
Gellissen, G. (2000). Applied Microbiology and Biotechnology, 54, 741–750.
Damasceno, L. M., Huang, C. J., & Batt, C. A. (2012). Applied Microbiology and Biotechnology, 93, 31–39.
Skropeta, D. (2009). Bioorganic & Medicinal Chemistry, 17, 2645–2653.
Jones, J., Krag, S. S., & Betenbaugh, M. J. (2005). Biochimica et Biophysica Acta-General Subjects, 1726, 121–137.
Celik, E., & Calik, P. (2012). Biotechnology Advances, 30, 1108–1118.
Gavel, Y., & Heijne, G. (1990). Protein Engineering, 3, 433–442.
Kasturi, L., Chen, H., & Shakin-Eshleman, S. H. (1997). Biochemical Journal, 323(Pt 2), 415–419.
Daly, R., & Hearn, M. T. (2005). Journal of Molecular Recognition, 18, 119–138.
Han, M. H., Wang, X. F., Ding, H. Y., Jin, M. Y., Wang, J. L., & Yu, X. B. (2014). Enzyme and Microbial Technology, 54, 32–37.
Han, M. H., Wang, X. F., Yan, G. L., Wang, W. X., Tao, Y., Liu, X., Cao, H., & Yu, X. B. (2014). Journal of Biotechnology, 171, 3–7.
Imperiali, B., & O’Connor, S. E. (1999). Current Opinion in Chemical Biology, 3, 643–649.
Macauley-Patrick, S., Fazenda, M. L., McNeil, B., & Harvey, L. M. (2005). Yeast, 22, 249–270.
Aebi, M. (2013). Biochimica et Biophysica Acta, 1833, 2430–2437.
Cereghino, J. L., & Cregg, J. M. (2000). FEMS Microbiology Reviews, 24, 45–66.
Dean, N. (1999). Biochimica et Biophysica Acta, 1426, 309–322.
Roth, J., Zuber, C., Park, S., Jang, I., Lee, Y., Kysela, K. G., Le Fourn, V., Santimaria, R., Guhl, B., & Cho, J. W. (2010). Molecules and Cells, 30, 497–506.
Tian, B., Chen, Y., & Ding, S. (2012). Protein Expression and Purification, 85, 44–50.
Brun, C., Monestier, O., Legardinier, S., Maftah, A., & Blanquet, V. (2012). Cellular Physiology and Biochemistry, 30, 791–804.
Hoshida, H., Fujita, T., Cha-aim, K., & Akada, R. (2013). Applied Microbiology and Biotechnology, 97, 5473–5482.
Muller-Steffner, H., Kuhn, I., Argentini, M., & Schuber, F. (2010). Protein Expression and Purification, 70, 151–157.
Wang, P., Zhang, J., Sun, Z., Chen, Y., & Liu, J. N. (2000). Protein Expression and Purification, 20, 179–185.
Ahmad, M., Hirz, M., Pichler, H., & Schwab, H. (2014). Applied Microbiology and Biotechnology, 7, 1–17.
Li, P., Anumanthan, A., Gao, X. G., Ilangovan, K., Suzara, V. V., Duzgunes, N., & Renugopalakrishnan, V. (2007). Applied Biochemistry and Biotechnology, 142, 105–124.
Acknowledgments
This work was supported by Open Project of Jiangsu Key Laboratory for Biomass-based Energy and Enzyme Technology (Grant No. JSBEET1306).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, M., Wang, W., Wang, X. et al. Enhanced Expression of Recombinant Elastase in Pichia pastoris through the Substitution of Thr for Ser in Asn-Xaa-Ser Sequons. Appl Biochem Biotechnol 175, 428–435 (2015). https://doi.org/10.1007/s12010-014-1284-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1284-5