Abstract
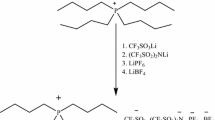
Ionic liquids (ILs) are being explored as solvents for the enzymatic methanolysis of triglycerides. However, most available ILs (especially hydrophobic ones) have poor capability in dissolving lipids, while hydrophilic ILs tend to cause enzyme inactivation. Recently, we synthesized a new type of ether-functionalized ionic liquids (ILs) carrying anions of acetate or formate; they are capable of dissolving a variety of substrates and are also lipase-compatible (Green Chem., 2008, 10, 696–705). In the present study, we carried out the lipase-catalyzed transesterifications of Miglyol® oil 812 and soybean oil in these novel ILs. These ILs are capable of dissolving oils at the reaction temperature (50 °C); meanwhile, lipases maintained high catalytic activities in these media even in high concentrations of methanol (up to 50% v/v). High conversions of Miglyol oil were observed in mixtures of IL and methanol (70/30, v/v) when the reaction was catalyzed by a variety of lipases and different enzyme preparations (free and immobilized), especially with the use of two alkylammonium ILs 2 and 3. The preliminary study on the transesterification of soybean oil in IL/methanol mixtures further confirms the potential of using oil-dissolving and lipase-stabilizing ILs in the efficient production of biodiesels.




Similar content being viewed by others
Notes
Most imidazolium ILs are based on anions of BF −4 , OTf−, MeSO −4 , Tf2N−, PF −6 , and SbF −6 . Poor yields were seen in most hydrophobic ILs except in [OMIM][Tf2N].
One unit hydrolyzes 1.0 microequivalent of fatty acid from triacetin in 1 h at pH 7.4 at 37 °C.
One unit is the amount of immobilized enzyme which forms 1% octyl laurate (GC, area percent) from 0.5 mmol lauric acid and 1.0 mmol 1-octanol in 10 mL water-saturated isooctane in 1 h at 20 °C.
One unit corresponds to the amount of enzyme which liberates 1 μmol oleic acid from triolein per minute at pH 7.5 and 37 °C.
References
Fukuda, H., Kondo, A., & Noda, H. (2001). Journal of Bioscience and Bioengineering, 92, 405–416.
Liu, B., & Zhao, Z. (2007). Journal of Chemical Technology and Biotechnology, 82, 775–780.
Du, W., Wang, L., & Liu, D. (2007). Green Chemistry, 9, 173–176.
Wang, W. G., Lyons, D. W., Clark, N. N., & Gautam, M. (2000). Environmental Science and Technology, 34, 933–939.
Schuchardt, U., Sercheli, R., & Vargas, R. M. (1998). Journal of the Brazilian Chemical Society, 9, 199–210.
Formo, M. W. (1954). Journal of the American Oil Chemists’ Society, 31, 548–559.
Freedman, B., Pryde, E. H., & Mounts, T. L. (1984). Journal of the American Oil Chemists’ Society, 61, 1638–1643.
Vicente, G., Martínez, M., & Aracil, J. (2004). Bioresource Technology, 92, 297–305.
Liu, Y., Lotero, E., Goodwin, J. G., & Lu, C. (2007). Journal of Catalysis, 246, 428–433.
Chai, F., Cao, F., Zhai, F., Chen, Y., Wang, X., & Su, Z. (2007). Advanced Synthesis & Catalysis, 349, 1057–1065.
Abreu, F. R., Alves, M. B., Macêdo, C. C. S., Zara, L. F., & Suarez, P. A. Z. (2005). Journal of Molecular Catalysis. A, Chemical, 227, 263–267.
Abreu, F. R., Lima, D. G., Hamú, E. H., Einloft, S., Rubim, J. C., & Suarez, P. A. Z. (2003). Journal of the American Oil Chemists’ Society, 80, 601–604.
Toda, M., Takagaki, A., Okamura, M., Kondo, J. N., Hayashi, S., Domen, K., et al. (2005). Nature, 438, 178.
DaSilveira Neto, B. A., Alves, M. B., Lapis, A. A. M., Nachtigall, F. M., Eberlin, M. N., Dupont, J., et al. (2007). Journal of Catalysis, 249, 154–161.
Lapis, A. A. M., de Oliveira, L. F., Neto, B. A. D., & Dupont, J. (2008). ChemSusChem, 1, 759–762.
Demirbas, A. (2005). Progress in Energy and Combustion Science, 31, 466–487.
Saka, S., & Kusdiana, D. (2001). Fuel, 80, 225–231.
Kusdiana, D., & Saka, S. (2001). Fuel, 80, 693–698.
He, H., Wang, T., & Zhu, S. (2007). Fuel, 86, 442–447.
Wu, Q., Chen, H., Han, M., Wang, D., & Wang, J. (2007). Industrial & Engineering Chemistry Research, 46, 7955–7960.
Han, M., Yi, W., Wu, Q., Liu, Y., Hong, Y., & Wang, D. (2009). Bioresource Technology, 100, 2308–2310.
Liang, X., Gong, G., Wu, H., & Yang, J. (2009). Fuel, 88, 613–616.
Akoh, C. C., Chang, S. W., Lee, G.-C., & Shaw, J.-F. (2007). Journal of Agricultural and Food Chemistry, 55, 8995–9005.
Zhao, X., El-Zahab, B., Brosnahan, R., Perry, J., & Wang, P. (2007). Applied Biochemistry and Biotechnology, 143, 236–243.
Shimada, Y., Watanabe, Y., Samukawa, T., Sugihara, A., Noda, H., Fukuda, H., et al. (1999). Journal of the American Oil Chemists’ Society, 76, 789–793.
Du, W., Xu, Y., Liu, D., & Zeng, J. (2004). Journal of Molecular Catalysis. B, Enzymatic, 30, 125–129.
Modi, M. K., Reddy, J. R. C., Rao, B. V. S. K., & Prasad, R. B. N. (2007). Bioresource Technology, 98, 1260–1264.
Iso, M., Chen, B., Eguchi, M., Kudo, T., & Shrestha, S. (2001). Journal of Molecular Catalysis. B, Enzymatic, 16, 53–58.
Royon, D., Daz, M., Ellenrieder, G., & Locatelli, S. (2007). Bioresource Technology, 98, 648–653.
van Rantwijk, F., & Sheldon, R. A. (2007). Chemical Reviews, 107, 2757–2785.
Ha, S. H., Lan, M. N., Lee, S. H., Hwang, S. M., & Koo, Y.-M. (2007). Enzyme and Microbial Technology, 41, 480–483.
Sunitha, S., Kanjilal, S., Reddy, P. S., & Prasad, R. B. N. (2007). Biotechnology Letters, 29, 1881–1885.
Gamba, M., Lapis, A. A. M., & Dupont, J. (2008). Advanced Synthesis & Catalysis, 350, 160–164.
Lau, R. M., Sorgedrager, M. J., Carrea, G., van Rantwijk, F., Secundo, F., & Sheldon, R. A. (2004). Green Chemistry, 6, 483–487.
Zhao, H., Baker, G. A., Song, Z., Olubajo, O., Crittle, T., & Peters, D. (2008). Green Chemistry, 10, 696–705.
Turner, M. B., Spear, S. K., Huddleston, J. G., Holbrey, J. D., & Rogers, R. D. (2003). Green Chemistry, 5, 443–447.
Toral, A. R., de los Rios, A. P., Hernández, F. J., Janssen, M. H. A., Schoevaart, R., van Rantwijk, F., et al. (2007). Enzyme and Microbial Technology, 40, 1095–1099.
de los Ríos, A. P., Hernández-Fernández, F. J., Martínez, F. A., Rubio, M., & Víllora, G. (2007). Biocatalysis and Biotransformation, 25, 151–156.
Moniruzzaman, M., Kamiya, N., & Goto, M. (2009). Langmuir, 25, 977–982.
Zhao, H., Baker, G. A., Song, Z., Olubajo, O., Zanders, L., & Campbell, S. M. (2009). Journal of Molecular Catalysis. B, Enzymatic, 57, 149–157.
Zhao, H., Jones, C. L., & Cowins, J. V. (2009). Green Chemistry, 11, 1128–1138. doi:10.1039/b905388c.
MacFarlane, D. R., Pringle, J. M., Johansson, K. M., Forsyth, S. A., & Forsyth, M. (2006). Chemical Communications, 1905–1917.
Liu, Q., Janssen, M. H. A., van Rantwijk, F., & Sheldon, R. A. (2005). Green Chemistry, 7, 39–42.
Watanabe, Y., Shimada, Y., Sugihara, A., Noda, H., Fukuda, H., & Tominaga, Y. (2000). Journal of the American Oil Chemists’ Society, 77, 355–360.
Soumanou, M. M., & Bornscheuer, U. T. (2003). Enzyme and Microbial Technology, 33, 97–103.
Köse, Ö., Tüter, M., & Aksoy, H. A. (2002). Bioresource Technology, 83, 125–129.
Yang, J.-S., Jeon, G.-J., Hur, B.-K., & Yang, J.-W. (2005). Journal of Microbiology and Biotechnology, 15, 1183–1188.
Babayan, V. K. (1981). Journal of the American Oil Chemists’ Society, 58, 49A–51A.
Flores, M. V., Naraghi, K., Engasser, J.-M., & Halling, P. J. (2002). Biotechnology and Bioengineering, 78, 815–821.
Acknowledgements
Acknowledgment is made to the Donors of the American Chemical Society Petroleum Research Fund (46776-GB1) for partial support of this research. The valuable and stimulating discussion with Dr. Jonathan H. Melman is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, H., Song, Z., Olubajo, O. et al. New Ether-Functionalized Ionic Liquids for Lipase-Catalyzed Synthesis of Biodiesel. Appl Biochem Biotechnol 162, 13–23 (2010). https://doi.org/10.1007/s12010-009-8717-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8717-6