Abstract
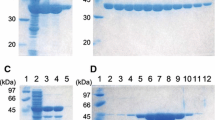
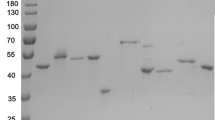
Although an X-ray model sequence of a leucine dehydrogenase from Bacillus sphaericus ATCC4525 was reported, the amino acid sequence of this enzyme has not been confirmed. In the current study, this leucine dehydrogenase gene was cloned, sequenced, and over-expressed in Escherichia coli, and the protein sequence has been clarified. This leucine dehydrogenase is not identical with that of B. sphaericus IFO3525 because there are 16 different amino acid residues between these two proteins. Since the information on the catalytic properties of leucine dehydrogenase from B. sphaericus ATCC4525 has been limited, the recombinant enzyme was purified as His-tagged protein and further studied. This enzyme showed activity toward aliphatic substrates for both oxidative deamination and reductive amination and is an effective catalyst for the asymmetric synthesis of α-amino acids from the corresponding α-ketoacids.


Similar content being viewed by others
References
Kataoka, K., & Tanizawa, K. (2003). Alteration of substrate specificity of leucine dehydrogenase by site-directed mutagenesis. Journal of Molecular Catalysis. B, Enzymatic, 23, 299–309. doi:10.1016/S1381-1177(03)00093-6.
Baker, P. J., Turnbull, A. P., Sedelnikova, S. E., Stillman, T. J., & Rice, D. W. (1995). A role for quaternary structure in the substrate specificity of leucine dehydrogenase. Structure (London), 3, 693–705.
Nagata, S., Tanizawa, K., Esaki, N., Sakamoto, Y., Ohshima, T., Tanaka, H., et al. (1988). Gene cloning and sequence determination of leucine dehydrogenase from Bacillus stearothermophilus and structural comparison with other NAD(P)+-dependent dehydrogenases. Biochemistry, 27, 9056–9062. doi:10.1021/bi00425a026.
Ohshima, T., Nishida, N., Bakthavatsalam, S., Kataoka, K., Takada, H., Yoshimura, T., et al. (1994). The purification, characterization, cloning and sequencing of the gene for a halostable and thermostable leucine dehydrogenase from Thermoactinomyces intermedius. European Journal of Biochemistry, 222, 305–312. doi:10.1111/j.1432-1033.1994.tb18869.x.
Katoh, R., Nagata, S., & Misono, H. (2003). Cloning and sequencing of the leucine dehydrogenase gene from Bacillus sphaericus IFO 3525 and importance of the C-terminal region for the enzyme activity. Journal of Molecular Catalysis. B, Enzymatic, 23, 239–247. doi:10.1016/S1381-1177(03)00086-9.
Lineweaver, H., & Burk, D. (1934). Determination of enzyme dissociation constants. Journal of the American Chemical Society, 56, 658–666. doi:10.1021/ja01318a036.
Turnbull, A. P., Ashford, S. R., Baker, P. J., Rice, D. W., Rodgers, F. H., Stillman, T. J., et al. (1994). Crystallization and quaternary structure analysis of the NAD+-dependent leucine dehydrogenase from Bacillus sphaericus. Journal of Molecular Biology, 236, 663–665. doi:10.1006/jmbi.1994.1176.
Ohshima, T., Misono, H., & Soda, K. (1978). Properties of crystalline leucine dehydrogenase from Bacillus sphaericus. The Journal of Biological Chemistry, 253, 5719–5725.
Katoh, R., Ngata, S., Ozawa, A., Ohshima, T., Kamekura, M., & Misono, H. (2003). Purification and characterization of leucine dehydrogenase from an alkalophilic halophile, Natronobacterium magadii MS-3. Journal of Molecular Catalysis. B, Enzymatic, 23, 231–238. doi:10.1016/S1381-1177(03)00085-7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Zhu, D., Hyatt, B.A. et al. Cloning, Protein Sequence Clarification, and Substrate Specificity of a Leucine Dehydrogenase from Bacillus sphaericus ATCC4525. Appl Biochem Biotechnol 158, 343–351 (2009). https://doi.org/10.1007/s12010-008-8304-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8304-2