Abstract
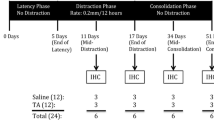
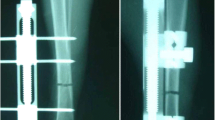
Previous reports suggest the application of exogenous BMPs can accelerate bone formation during distraction osteogenesis (DO). However, there are drawbacks associated with the use of exogenous BMPs. A possible alternative to the use of exogenous BMPs is to upregulate the expression of endogenous BMPs. Since DO results in spontaneously generated de novo bone formation in a uniform radiographic, histological, and biomechanical temporal sequence, a genetically engineered model lacking endogenous BMP2 should have measurable deficits in bone formation at different time points. We performed DO on BMP2 fl/+ and BMP2 fl/+ cre mice using a miniature Ilizarov fixator. Distracted samples were collected at various time points and analyzed using Real Time-quantitative PCR, μCT, radiology, immunohistochemistry, histology, and biomechanical testing. Immunohistochemical studies of 34-day heterozygous samples showed reduced expression of BMP2, BMP7, BMPR1a, ACTR1, and ACTR2b. μCT analysis of 51-day heterozygous samples revealed a decrease in trabecular number and increase in trabecular separation. Biomechanical testing of 51-day heterozygous samples revealed decreased stiffness and increased ultimate displacement. Radiological analysis showed the heterozygotes contained a decreased bone fill score at 17, 34, and 51 days. These data suggest endogenous BMPs are important for bone healing and manipulating endogenous BMPs may help accelerate bone consolidation during DO.







Similar content being viewed by others
References
Abbaspour A, Takata S, Sairyo K, Katoh S, Yukata K, Yasui N. Continuous local infusion of fibroblast growth factor-2 enhances consolidation of the bone segment lengthened by distraction osteogenesis in rabbit experiment. Bone. 2008;42:98–106.
Axelrad T, Steen B, Lowenberg D, Creevy W, Einhorn T. Heterotopic ossification after the use of commercially available recombinant human bone morphogenetic proteins in four patients. J Bone Joint Surg Br. 2008;90:1617–1622.
Birch J, Samchukov M. Use of the Ilizarov method to correct lower limb deformities in children and adolescents. J Am Acad Orthop Surg. 2004;12:144–154.
Casap N, Venezia N, Wilensky A, Samuni Y. VEGF facilitates periosteal distraction-induced osteogenesis in rabbits: a micro-computerized tomography study. Tissue Eng Part A. 2008;14:247–253.
Colburn N, Zaal K, Wang F, Tuan R. A role for/T cells in a mouse model of fracture healing. Arthritis Rheum. 2009;60:1694–1703.
Fowlkes J, Thrailkill K, Liu L, Wahl E, Bunn R, Cockrell G, Perrien D, Aronson J, Lumpkin Jr C. Effects of systemic and local administration of recombinant human IGF-I (rhIGF-I) on de novo bone formation in an aged mouse model. J Bone Miner Res. 2006;21:1359–1366.
Friedlaender G, Perry C, Cole J, Cook S, Cierny G, Muschler G, Zych G, Calhoun J, LaForte A, Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83:S151–S158.
Giannoudis P, Kanakaris N, Einhorn T. Interaction of bone morphogenetic proteins with cells of the osteoclast lineage: review of the existing evidence. Osteoporos Int. 2007;18:1565–1581.
Govender S, Csimma C, Genant H, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Börner M, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Rüter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T. BMP-2 Evaluation in Surgery for Tibial Trauma (BESTT) Study Group. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84:2123–2134.
Haque T, Hamade F, Alam N, Kotsiopriftis M, Lauzier D, St-Arnaud R, Hamdy RC. Characterizing the BMP pathway in a wild type mouse model of distraction osteogenesis. Bone. 2008;42:1144–1153.
Haque T, Mandu-Hrit M, Rauch F, Lauzier D, Tabrizian M, Hamdy RC. Immunohistochemical localization of bone morphogenetic protein-signaling Smads during long-bone distraction osteogenesis. J Histochem Cytochem. 2006;54:407–415.
Hsu W, Wang J. The use of bone morphogenetic protein in spinal fusion. Spine J. 2008;8:419–425.
Hu J, Li J, Wang D, Buckley M, Agarwal S. Differences in mandibular distraction osteogenesis after corticotomy and osteotomy. Int J Oral Maxillofac Surg. 2002;31:185–189.
Hu J, Qi M, Zou S, Li J, Luo E. Callus formation enhanced by BMP-7 ex vivo gene therapy during distraction osteogenesis in rats. J Orthop Res. 2007;25:241–251.
Ilizarov G. The tension-stress effect on the genesis and growth of tissues. I: The infleunces of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989;238:249–281.
Ilizarov G. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989;239:263–285.
Lammens J, Liu Z, Luyten F. Bone morphogenetic protein signaling in the murine distraction osteogenesis model. Acta Orthop Belg. 2009;75:94–102.
Lammens J, Nijs J, Schepers E, Ectors N, Lismont D, Verduyckt B. The effect of bone morphogenetic protein-7 (OP-1) and demineralized bone matrix (DBM) in the rabbit tibial distraction model. Acta Orthop Belg. 2009;75:103–109.
Lloyd S, Yuan Y, Kostenuik P, Ominsky M, Lau A, Morony S, Stolina M, Asuncion F, Bateman T. Soluble RANKL induces high bone turnover and decreases bone volume, density, and strength in mice. Calcif Tissue Int. 2008;82:361–372.
Mandu-Hrit M, Haque T, Lauzier D, Kotsiopriftis M, Rauch F, Tabrizian M, Henderson J, Hamdy RC. Early injection of OP-1 during distraction osteogenesis accelerates new bone formation in rabbits. Growth Factors. 2006;24:172–183.
Moore D, Ehrlich M, McAllister S, Machan J, Hart C, Voigt C, Lesieur-Brooks A, Webber E. Recombinant human platelet-derived growth factor-BB augmentation of new-bone formation in a rat model of distraction osteogenesis. J Bone Joint Surg Am. 2009;91:1973–1984.
Namdari S, Wei L, Moore D, Chen Q. Reduced limb length and worsened osteoarthritis in adult mice after genetic inhibition of p38 MAP kinase activity in cartilage. Arthritis Rheum. 2008;58:3520–3529.
Okamoto M, Murai J, Yoshikawa H, Tsumaki N. Bone morphogenetic proteins in bone stimulate osteoclasts and osteoblasts during bone development. J Bone Miner Res. 2006;21:1022–1033.
Ozkan K, Eralp L, Kocaoglu M, Ahishali B, Bilgic B, Mutlu Z, Turker M, Ozkan F. The effect of transforming growth factor 1 (TGF-1) on the regenerate bone in distraction osteogenesis. Growth Factors. 2007;25:101–107.
Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Rel Res 1990;250:81–104.
Rengachary S. Bone morphogenetic proteins: basic concepts. Neurosurg Focus. 2002;13:e2.
Shimizu T, Jayawardana B, Nishimoto H, Kaneko E, Tetsuka M, Miyamoto A. Involvement of the bone morphogenetic protein/receptor system during follicle development in the bovine ovary: Hormonal regulation of the expression of bone morphogenetic protein 7 (BMP-7) and its receptors (ActRII and ALK-2). Mol Cell Endocrinol. 2006;249:78–83.
Tay B, Le A, Gould S, Helms J. Histochemical and molecular analyses of distraction osteogenesis in a mouse model. J Orthop Res. 1998;16:636–642.
Troulis M, Coppe C, O’Neil MJ, Kaban LB. Ultrasound: assessment of the distraction osteogenesis wound in patients undergoing mandibular lengthening. J Oral Maxillofac Surg. 2003;61:1144–1149.
Tsuji K, Bandyopadhyay A, Harfe B, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin C, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429.
Vaibhav B, Nilesh P, Vikram S, Anshul C. Bone morphogenic protein and its application in trauma cases: A current concept update. Injury. 2007;38:1227–1235.
Wysocki R, Cohen M. Ectopic ossification of the triceps muscle after application of bone morphogenetic protein-7 to the distal humerus for recalcitrant nonunion: a case report. J Hand Surg Am. 2007;32:647–650.
Zhang H. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986.
Acknowledgements
We thank members of Dr. St-Arnaud’s lab, Fares Hamade, Tasima Haque, Maria Kotsioprifitis, and Noémi Dahan for assisting with the study, Dr. Vicki Rosen (Harvard School of Medicine, Boston, MA) for the conditional BMP2 knockout mice, Guylaine Bedard and Mark Lepik for help with figures, and the McGill Bone Centre for their assistance with the radiology/μCT and biomechanical testing analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownerships, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article. This work was supported by Shriners of North America operating grant no. 8700.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Shriners Hospital for Children and the Montréal Children’s Hospital of McGill University, Montréal, QC, Canada.
About this article
Cite this article
Alam, N., St-Arnaud, R., Lauzier, D. et al. Are Endogenous BMPs Necessary for Bone Healing during Distraction Osteogenesis?. Clin Orthop Relat Res 467, 3190–3198 (2009). https://doi.org/10.1007/s11999-009-1065-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-009-1065-6