Abstract
Recent decades have seen the development of many effective and innovative technologies for extra virgin olive oil (EVOO) extraction. Various solutions have been proposed to remove dissolved oxygen from the oil. Given these issues, we have designed and developed a system that can be added to the centrifuges that are already used in the olive oil industry. The system reduces the oxidative impact through the release of a technical gas inside the separator, and consequently delays the onset of defects related to oxidation. The experiment tested different N2 flow rates, directly into the vertical centrifuge, and four levels of N2 were tested–a control level (no N2 injection); low (20 L/min), medium (40 L/min), and maximum (80 L/min)–in order to evaluate the effectiveness of this new technique on EVOO quality. This experiment demonstrates that the objectives have been achieved. The EVOO produced using our system had lower dissolved oxygen content with N2 injection, along with an enriched volatile fraction, and higher biophenol concentrations. The chemical analyses were confirmed by a sensory analysis, with an increase in fruity intensity and bitter taste.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extra virgin olive oil (EVOO) is obtained from olives by mechanical means. It is one of the few vegetable oils that can be consumed without refining, and is an important contributor to the economy, especially in Mediterranean countries. Consumption is increasing worldwide, due to its nutritional value and characteristic aroma, together with the presence of a large number of beneficial chemical compounds (Morrone et al., 2017; Pérez‐Jiménez et al., 2007). The best EVO oils are obtained by the correctly application of the best practices operative, for example, by early harvesting the drupes, working the product promptly, reducing the process water as much as possible, and limiting the heating of the olive pasta to be able to maintain a content of bioactive phenolic substances useful for the application of the health claim (Bellumori et al., 2019).
EVOO quality is closely related to the extraction process (Di Giovacchino et al., 2002; Fregapane & Salvador, 2013; Guerrini et al., 2017). Extraction consists of crushing olives to a paste, which is malaxated and centrifuged, resulting in an oily must (oil that contains small amounts of residual vegetative water and impurities). The must requires further cleaning, which is performed by washing the oil in a vertical centrifuge (Masella et al., 2009).
EVOO producers aim to guarantee that product quality remains stable over time. Typical operations to achieve this include vertical centrifugation and filtration. Filtration, when applied, is an efficient way to completely remove water in emulsion and solids in suspension; together with other operations, it transforms the appearance of the oil from veiled to limpid (Fortini et al., 2016; Fregapane et al., 2006; Guerrini et al., 2020a, b).
Olive paste is kneaded during malaxation to increase extractability, before oil is separated from the pomace with a decanter centrifuge (Inarejos-García et al., 2011; Trapani et al., 2017) in consecutive steps: horizontal centrifugation separates the water and pomace, while vertical centrifugation clarifies the oil by removing part of the suspended solids and water (Guerrini et al., 2018).
The current standard method used to finish and clarify the oil is vertical or disc centrifugation at 5000–7000 rpm (Baccioni & Peri, 2014). This method is preferred because it has high operating capacity and requires a limited workforce. However, several studies have established that using a vertical centrifuge after decanting allows oxygen to dissolve into the olive oil. The increase in dissolved oxygen is reflected in a worsening of the oxidative state, measured as a significant increase in the peroxide value. Oxidation shortens the product’s shelf-life, due to a decrease in antioxidant compounds and biophenols and an increase in oxidation‐related parameters–moreover, it can compromise EVOO quality due to the presence of the rancid defect (Masella et al., 2009; Rodis et al., 2002; EFSA Panel on Dietetic Products and Allergies, 2011).
Oxidative stability is an important indicator of EVOO quality and its shelf-life (Hamilton & Allen, 1994; Silva et al., 2001). Low molecular weight, off-flavor compounds are produced during oxidation, which can make the oil less acceptable (or even unacceptable) to consumers, or unsuitable for industrial use as a food ingredient (Choe & Min, 2006). Autoxidation of food lipids affects molecules with one or more allyl groups, via a free-radical mechanism, and, once degradation begins, reactions with the formed hydroperoxides significantly increase the number of volatile oxygenated compounds (Choe & Min, 2006). As EVOO is mainly composed of unsaturated fatty acids, rancidity can occur during storage. The rancid attribute is a widely studied sensory defect. It is due to lipid auto-oxidation molecules, generally heptane, E-2-heptenal, 2,4-heptadienal, 2-heptanol, nonanal, 2,4-nonadienal, and decanal volatile compounds (Kalua et al., 2007; Morales et al., 2005). Finally, it is well-known that EVOO antioxidant phenolic compounds (notably secoiridoids) are able to slow down the formation of the rancid defect (Angerosa et al., 2004).
Recent decades have seen the development of many effective and innovative technologies for EVOO extraction (Clodoveo, 2013). Various solutions have been proposed to remove dissolved oxygen from the oil. One example is the rapid removal of oxygen using a nitrogen stripping technique (Guerrini et al., 2018). Another example in the food industry is the use of centrifuges with inert technical gases. However, these techniques are not widely used in EVOO production, due to the cost, and cost is the reason why the olive oil industry uses open centrifuges, in which the oil is in continuous contact with the air. Given these issues, we have tested a system that can be added to the centrifuges that are already used in the olive oil industry. The system reduces the oxidative impact through the release of a technical gas inside the separator, and consequently delays the oxidation.
This system could protect EVOO quality during the two centrifugations, which increase both the shelf-life of the oil, and the period during which it can be marketed as extra virgin. It aims to avoid or reduce the addition of dissolved oxygen to the EVOO, reducing the oxidation of phenolic compounds and unsaturated fatty acids present in the product, thus delaying the potential appearance of the rancid defect, causing it to downgrade. As the device can be installed on open vertical separators, there is no need to replace machines currently in use, and existing open equipment is able to perform as well as the closed separators already on the market.
This study reports the results of tests of the performance of the system, at different gas flow rates.
Materials and Methods
The Gas Injection Device, Design, and Implementation
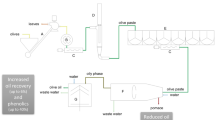
A device was designed and built to simulate a real industrial process. The device, which allows the introduction of a technical gas, can be added to most existing centrifuges as an accessory. The gas selected for the experimental trials was nitrogen. Nitrogen (N2) is widely used, pure or mixed, to preserve industrial food products. The principal mechanism is the replacement of O2 in the headspace (Mannheim & Soffer, 1996). N2 is available in cylinders or can be produced directly by the company. The device, reported in Fig. 1, consists of the following:
-
A system that seals the rotation chamber of the centrifuge
-
A 3-way connection for the introduction of water, the olive oil to be treated, and the technical gas
-
Appropriate systems for measuring and regulating the flow rates of the three fluids
The apparatus can modulate the entry of the three working elements to the centrifuge–in particular, oil coming from the decanter, water (necessary for correct operation), and the technical gas used to prevent contact between the oil and the air–and thus reduce the concentration of dissolved oxygen.
Olives
Olive (Olea europaea) cultivar Frantoio were manually picked in Tavarnelle Val di Pesa (Firenze, Italy, approx. 43° 33′ N/11° 10′ E) in early November 2020. Fruits were in good sanitary/physical condition (assessed by visual inspection by company technicians), with no signs of insect or pest infestation, or mechanical damage. The ripening index was assessed as 4 (Uceda & Frias, 1975), the exterior color of the skin was consistently almost entirely purple or black, while the flesh was white.
Experimental Conditions
The pilot device was tested in experiments carried out in an industrial-scale olive oil extraction plant. The experiment tested different N2 flow rates, directly into the vertical centrifuge. Trials were conducted in November 2020 at Tavarnelle Val di Pesa, Firenze (Italy). A homogeneous batch of 3000 kg of Frantoio cultivar was split into sub-batches, in order to test four levels of gas injection in three replicates.
Olives were de-leafed, washed, and milled with a continuous crusher (theoretical capability 2500 kg/h) composed of a hummer/blade crusher working at 2800 rpm, followed by a 4000 kg/h malaxer (MORI-TEM srl Via Leonardo da Vinci 59, 50028 Barberino Tavarnelle (FI), Italy). The paste obtained was first malaxed for 20 min at 25 °C, and then sent, at a flow rate of 1800 kg/h, to a two-phase horizontal centrifuge which separated the oil from the water and pomace at a rate of 750 kg/h, and a liquid/ liquid vertical centrifuge with a capability of 1500/1800 kg/h. The oily must was cleaned by a vertical centrifuge operating at 6500 rpm, fed with 15 L tap water/h.
Four levels of N2 were tested: a control level (no N2 injection); low (20 L/min), medium (40 L/min), and maximum (80 L/min). All trials were performed in triplicate, making a total of 12 samples. After gas injection, the oxygen concentration was measured. Control samples were also monitored.
Finally, the oil that was produced was immediately filtered using a filter press.
Olive Oil Analysis
Oil samples obtained from trials were analyzed for several parameters.
Dissolved oxygen concentration (mg/L) was measured at the same olive oil temperature (20 °C) by a portable oxygen analyzer model InPro 6850i (Mettler-Toledo S.p.A, Italy) and with calibration of the instrument with reference to the atmospheric pressure before each new measurement (Masella et al., 2009).
Turbidity was measured in nephelometric turbidity units (NTU), using a Hach Model 2100 turbidimeter (Hach, Loveland, CO). About 25 g of sample oil was taken from a bottle that had been shaken for 1 min, and put in a standard clean glass vessel, which was then inserted into the closed vessel chamber of the turbidimeter. Turbidity was measured at equilibrium after approximately 1 min.
Water content (% w/w) was calculated by weighing the difference after 10 g of olive oil was dried for 24 h at 105 °C. The effects of the treatment were evaluated immediately post‐production, and after 6 months of storage. For shelf-life tests, each oil pair was sampled in triplicate, stored in a separate dark can, at room temperature in the dark.
Free fatty acids (% oleic acid), peroxide value (meq O2 per kg of oil), and UV spectroscopic indices (K232, K268, and ΔK) according to official methods (EC, 2008) have been determined.
Biophenolic fractions were extracted and identified following the International Olive Council (IOC) official method (International Olive Council, 2017). Phenolic compounds were extracted using a methanol:water 80:20 (v/v) solution. HPLC analysis was performed using a HP 1100 coupled with both a DAD and MS detector, the latter equipped with an HP1100 MSD API-electrospray interface (Agilent Technologies, Palo Alto, CA, USA). A Poroshell 120 EC-C18 column (150 mm × 3.0 mm id, 2.7-μm particle size; Agilent Technologies, Palo Alto, CA, USA) was used for separation. According to the official method, acetonitrile, H2O, and methanol were adopted as elution solvents following the elution gradient described by the IOC. The chromatogram was recorded at 280 nm, using syringic acid as internal standard, while the phenolic concentration was expressed as mg kg−1 of tyrosol.
Identification and quantification of VOCs were performed by headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry (HS–SPME–GC–MS) using the multiple internal standard method, as described by Fortini et al. (2017).
Analyses involved weighing 4.3 g of an oil sample and 0.1 g of an internal standard (ISTD mix) into 20-mL screw cap vials fitted with a PTFE/silicone septum. After 5-min equilibrium at 60 °C, a SPME fiber (50/30 µm DVB/CAR/PDMS, Supelco, St. Louis, USA) was exposed for 20 min in the vial headspace under orbital shaking (500 rpm). Then, the fiber was immediately desorbed for 2 min in a gas chromatograph injection port operating in splitless mode at 260 °C.
Compounds were identified and quantified (mg/kg) by comparison of their mass spectra and retention times with those of the ISTD mix, consisting of the following 11 compounds: 3,4-dimethylphenol, 4-methyl-2-pentanol, hexanoic acid-d11, 1-butanol-d10, ethyl acetate-d8, toluene-d8, ethyl hexanoate-d11, acetic acid 2,2,2-d3, 6-chloro-2-hexanone, 3-octanone, and trimethyl acetaldehyde. The same amount of ISTD mix was added to calibration scales to normalize each analyte concentration of the calibration curve to that of the respective ISTD mix.
GM-MS identification of VOCs was performed using a Trace CG-MS Thermo Fisher Scientific, equipped with a ZB-FFAP capillary column (Zebron) 30 m × 0.25 mm ID, 0.25 µm df. The temperature of the column was controlled as follows: 36 °C for 10 min, increase to 156 °C at 4 °C per min, increase to 260 °C at 10 °C per min, decrease to 250 °C at 10 °C per min, with a hold time of 2 min. Helium was used as the carrier gas at a constant flow of 0.8 mL/min. The temperature of both the ion source and transfer line was 250 °C. The mass detector operated in scan mode within a 30–330 Th mass range at 1500 Th s−1, with an ionization energy of 70 eV.
VOC quantification was carried out by comparing each mass spectra and retention time with those of injected authentic standards. The stock external standard mix contained 71 analytes in refined oil, which was previously verified to be free of any interferent. The analytes and their concentration ranges were chosen based on previous works on Italian virgin olive oils.
Sensory Analysis
Sensory evaluation of EVOO samples was performed by a panel of eight assessors, who had been trained according to the IOC’s method for organoleptic assessment, which is described in EEC regulations (EC, 2008). Sensory evaluation was performed in three separate sessions, and samples were randomized between assessors.
Statistical Analysis
A two-way ANOVA tested for significant differences between the samples treated (or not) with N2, at three injection rates, and two storage times. The significance level was set to p < 0.05. The post hoc Tukey HSD test was applied to assess differences among means, where appropriate. All statistical analyses were performed using the R software package (version 3.6.2).
Results and Discussion
A device that enables the injection of a technical gas during EVOO centrifugation was tested in an industrial plant, in order to demonstrate its applicability and establish the effects of this system on EVOO quality.
Physical and Chemical Characterization of Samples
All olive oil samples were immediately characterized in order to evaluate the initial effects of the device. As reported in Table 1, dissolved oxygen was the first parameter to be monitored in the produced EVOO, with and without treatment. As expected, we found a significant difference between treatments. There was a decrease in the concentration of dissolved oxygen as a function of the increase in gas flow rate. It was particularly important to monitor this parameter to validate the proposed solution, notably because controlling the concentration of dissolved oxygen can limit oxidative damage to the EVOO. Turbidity fell from 1117.2 ± 86 NTU to 555.2 ± 62 NTU, and mean moisture was 0.20 ± 0.03. These results are consistent with the literature (Breschi et al., 2019; Guerrini et al., 2020a, b). The trend observed was that the turbidity decreased in relation to the addition on the inert gas. This result is confirmed by a patented solution reported in scientific paper (Lozano-Sánchez et al., 2010), which have demonstrated that adding a constant flow of inert gas directly to the center of the olive oil mass generates a circular movement of the oil mass which facilitates the precipitation of suspended solids (Lozano-Sánchez et al., 2010).
The ANOVA found significant differences between the following: samples treated with gas and control samples; samples treated at different gas flow rates; and between the two storage times. The interaction between the two tested variables (gas treatment and storage time) did not affect the chemical parameters reported in Table 2. On the other hand, main effects were observed for each of the two variables.
The analysis found that decarboxymethyl oleuropein aglycone dialdehyde (3,4-DHPEA-EDA) was the most abundant biophenol (roughly 35% of total biophenols); together with oleuropein aglycone dialdehyde (3,4-DHPEA-EA), it is the major contributor to olive oil oxidative stability (Servili et al., 2014; Veneziani et al., 2017). The results showed that its concentration was very sensitive to the gas injection, not only when comparing treated and control samples, but also between samples treated at different gas flow rates. This trend remained after 6 months of storage. Average 3,4-DHPEA-EDA content of untreated oil (i.e., samples that were filtered directly after separation with no N2 addition) was 25 mg/kg, compared to a mean total of 67 mg/kg for samples treated with N2. Concentrations increased as a function of the increase in N2 flow rate, reaching a maximum of about 88 mg/kg at 80 L/min.
The ligstroside derivative p-HPEA-EDA followed the same trend. Concentrations increased as the N2 flow rate increased and ranged from a minimum of 33.8 mg/kg (at 20 L/min) to a maximum of 51 mg/kg (at 80 L/min). The lowest value was found for the control sample, as reported in Table 2. Aglycone secoiridoids such as 3,4-DHPEA-EDA, p-HPEA-EDA, p-HPEA-EA, and 3,4-DHPEA-EA appear during crushing due to the hydrolysis of oleuropein, dimethyl-oleuropein, and ligstroside. The experiment found that the content of oleuropein, ligstroside, and their derivatives was proportional to the intensity of bitterness and pungency. Statistically significant differences were found for total biophenols (p = 0.001), as a function of the addition of N2. In particular, biophenol concentration increased in treated samples, and as a function of the increase in gas flow rate.
The result confirms that the modified vertical centrifuge can modulate olive oil characteristics, especially compounds related to sensorial characteristics, such as phenols, as reported in different studies (Funes et al., 2018; Masella et al., 2009). Earlier work has demonstrated that varying the oxygen concentration during processing could be a strategy to optimize the phenolic concentration in EVOO. In several operative situations, oxygen can be regulated during malaxation either by treatment with inert gases, or using the CO2 that is naturally produced by the olive paste (Clodoveo, 2012). Inert gases like N2 and Ar have been used with sealed malaxers to increase antioxidant activity and extend the shelf-life of EVOO (Vierhuis et al., 2001), while other studies have found that the sterol and fatty acid composition of oil was not affected by N2 application (Yorulmaz et al., 2011).
It has been demonstrated that oleuropein, demethyloleuropein, and ligstroside derivatives are very sensitive to the oxygen concentration during malaxation (Migliorini et al., 2006). The latter result is in agreement with Masella et al. (2011), who confirmed that reduced oxygen concentrations produced oils that were characterized by less oxidation and greater antioxidant concentration.
A clear trend was observed for the peroxide number, which was influenced by both the gas treatment and storage time. This parameter played a key role in the differences observed (F related to storage time = 350.7, compared to F related to the gas treatment = about 7.9). While, in general, it increased over time, the rate of increase was slower in treated samples, especially samples treated at higher flow rates. This result was very interesting and was confirmed by the trend observed for the concentration of antioxidant compounds. It demonstrates the effectiveness of the treatment in not only increasing the concentration of positive compounds, but also during storage. Compound concentrations did not decrease, and the resulting oil had less oxidative damage than control samples.
Some compound concentrations were only affected by storage time. Specifically, concentrations of oleuropein, decarboxymethyl oleuropein aglycone oxidized dialdehyde, and oleuropein aglycone, aldehyde and hydroxylic, decreased as a function of storage time. This decrease was presumably due to their hydrolysis, as a correlation with an increase in tyrosol and 5-hydroxytyrosol was noted.
VOC Concentrations as a Function of Gas Treatment and Storage Time
The analysis identified a significant increase in some VOCs as a function of the gas treatment and storage time, but no interaction between the two parameters. Several statistically significant differences (p < 0.05) were identified in the LOX pathway. In particular, Table 3 shows that significant differences were found for 1-penten-3-ol, E-2-hexenal, Z3-hexen-1-ol, and E2-esenol, and the sum of C5 and C6 compounds, due to the increased gas flow rate during separation. In these cases, concentrations were higher in treated samples, but storage time had no effect. Furthermore, samples treated at the higher flow rate had higher concentrations of these compounds, which have a positive effect on perceived aroma and flavor.
The greatest difference was observed for E-2-hexenal. This compound provides the characteristic “green” note of olive oil, and is the most abundant C6 aldehyde, representing about 90% of C6 compounds. It is a product of the LOX pathway, and is inversely related to the degree of oxidation and maturity of VOO (Kalua et al., 2007). Concentrations in control samples were about 13.8 mg/kg; at the minimum tested level of gas injection, this increased to 17 mg/kg, and reached a maximum concentration of 21.6 mg/kg for samples treated at the highest flow rate (80 L\min). As the odor threshold is 0.25 mg/kg, this compound was clearly perceivable in all samples. Concentrations for samples treated at 40 L/min were between the other two levels, at around 18.5 mg/kg. E-2-hexenal is described as having “green leaves” and “green and sweet” sensory notes (Aparicio & Luna, 2002), and its low odor threshold means that it is one of the most important VOCs in the LOX pathway (Polari et al., 2018), along with several others that contribute to the fruity attribute.
These results are in good agreement with Tamborrino et al. (2014), who saturated the malaxer with N2, and injected air continuously into the olive paste. The latter study also observed an increase in E-2-hexenal and Z-3-hexenal compounds.
The experiment did not detect an effect on compounds defined as unpleasant. Only one significant difference was found (for decanal), and the observed trend was in the opposite direction. The highest concentration was found for control samples, while there was no difference for treated samples (or N2 flow rates), and the concentration was lower.
Considering total VOC concentrations from C6 and C5 branches, we found a statistically significant lower concentration in control samples (no N2 addition) compared to treated samples. The gas treatment had a significant effect on fruity compound concentrations, especially for C6 compounds, and the analysis found that 80 L/min was the value that has generate major increment, thus to the highest flow rate.
Another interesting result relates to compounds that are perceived as unpleasant. Here, no significant differences were found as a function of the gas treatment. Overall concentrations were very low with respect to positive attributes, and were stable independent of the operative condition.
Finally, a few compounds were influenced by the storage time. Heptanal and 2-heptanol concentrations, in particular 1-hexanol, fell during storage, independent of the gas treatment. This compound is related to green notes.
Sensory Analysis as a Function of Gas Treatment and Storage Time
A sensory test was run to assess whether the observed increase in concentrations (especially of compounds associated with positive notes) was perceptible to the consumer. Here, the aim was to understand if our proposed solution could increase (desirable) perceptions of green notes, and, more importantly, avoid the appearance of rancidity. Table 4 reports the intensity of the main descriptors. Overall, judges were unable to detect any sensory defects in any of the samples. The sensory evaluation found a value of 0 for all defect attributes (except one sample), and values greater than 0 for fruity attributes.
The N2 treatments influenced the perception of fruity and bitterness. Table 4 shows that for these two attributes, control samples (independent of storage time) were perceived as less bitter and less fruity, while samples treated at the maximum flow rate were perceived as more fruity and more bitter. This result confirms the trend observed in the VOC analysis. The mean fruity intensity registered for samples treated at high N2 levels was 5.4, compared to 3.85 for the other samples (on a 10-point scale). Here again, 40 L/min was the critical flow rate that induced a change in fruity perception. Increased bitterness was detected not only for treated samples compared to control samples, but also between treatments. An interaction between gas treatment and storage time was found for the pungent attribute; intensity increased both in the treatment condition, and after storage.
These sensory results confirm the chemical analyses. Samples treated with N2 had higher concentrations of several biophenolic compounds, and bitterness and pungency are mainly related to the quali-quantitative presence of phenolic compounds in EVOO (Genovese et al., 2020). A strong correlation between secoiridoid concentration and bitterness and pungency has been observed in previous studies (García et al., 2001; Tovar et al., 2001). Furthermore, Andrewes et al. (2003) reported that bitterness is likely to be a common feature of the majority of VOO phenols, but that the key source of pungency is probably p-HPEA-EDA. These results confirm these earlier findings, as we found higher concentrations of these compounds in samples perceived as more bitter and more pungent.
Results for the negative rancid attribute were different. Rancid was only perceived by the panelists for the control sample after 6 months of storage. An interaction was found between storage time and gas treatment, reflected in the appearance of this negative attribute. The presence of this attribute compromised the oil’s quality, and, consequently, its product class.
Conclusions
This study reports the results of an evaluation of one system to consistently reduce the addition of oxygen dissolved in olive oil during the production process. The aim was to create an apparatus that makes it possible to obtain an olive oil that has a longer shelf-life than the oils currently on the market, and, therefore, to increase the period during which it can be marketed as EVOO. This device can be easily adapted to the industrial scale and has been shown to be technically feasible. The system has been tested with different flow rate configurations and N2 dosage.
This experiment demonstrates that the objectives can be achieved. The EVOO produced using this system had lower dissolved oxygen content with N2 injection, and the turbidity value lower at the maximum gas dosage, along with an enriched volatile fraction, and higher biophenol concentrations. The chemical analyses were confirmed by a sensory analysis, with an increase in fruity intensity and bitter taste.
Concerning storage time, after 6 months of storage, treated samples had high biophenol content, specifically secoiridoid derivatives. Storage also affected a few other compounds, including oleuropein, and some volatile compounds. There was an important interaction between the gas treatment and storage time, observed by the sensory test. Notably, the rancid defect was detected in the control sample after 6 months of storage. This result demonstrates that the application of an inert gas during the separation stage could support the development of positive notes and mitigate the appearance of defects.
This innovative device can be easily configured to produce EVOO with specific characteristics that are a function of operational conditions, and with better, more diverse chemical and organoleptic characteristics.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Andrewes, P., Busch, J. L. H. C., De Joode, T., Groenewegen, A., & Alexandre, H. (2003). Sensory Properties of Virgin Olive Oil Polyphenols: Identification of Deacetoxy-Ligstroside Aglycon as a Key Contributor to Pungency. Journal of Agricultural and Food Chemistry, 51(5), 1415–1420. https://doi.org/10.1021/jf026042j
Angerosa, F., Servili, M., Selvaggini, R., Taticchi, A., Esposto, S., & Montedoro, G. (2004). Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. Journal of Chromatography A, 1054(1–2), 17–31. https://doi.org/10.1016/j.chroma.2004.07.093
Aparicio, R., & Luna, G. (2002). Characterisation of monovarietal virgin olive oils. European Journal of Lipid Science and Technology, 104(9–10), 614–627.
Baccioni, L., & Peri, C. (2014). Centrifugal separation. The extra-virgin. Olive oil handbook, Ltd (pp. 139–154). John Wiley.
Bellumori, M., Cecchi, L., Innocenti, M., Clodoveo, M. L., Corbo, F., & Mulinacci, N. (2019). The EFSA health claim on olive oil polyphenols: Acid hydrolysis validation and total hydroxytyrosol and tyrosol determination in Italian virgin olive oils. Molecules, 24(11), 2179. https://doi.org/10.3390/molecules24112179
Breschi, C., Guerrini, L., Domizio, P., Ferraro, G., Calamai, L., Canuti, V., et al. (2019). Physical, chemical, and biological characterization of veiled extra virgin olive oil turbidity for degradation risk assessment. European Journal of Lipid Science and Technology, 121(11), 1900195. https://doi.org/10.1002/ejlt.201900195
Choe, E., & Min, D. B. (2006). Comprehensive reviews in food science and food safety mechanisms and factors for edible oil oxidation. Comprehensive Reviews in Food Science and Food Safety, 5, 169–186.
Clodoveo, M. L. (2012). Malaxation: Influence on virgin olive oil quality. Past, present and future – An overview. Trends in Food Science & Technology, 25(1), 13–23. https://doi.org/10.1016/J.TIFS.2011.11.004
Clodoveo, M. L. (2013). New advances in the development of innovative virgin olive oil extraction plants: Looking back to see the future. Food Research International, 54(1), 726–729. https://doi.org/10.1016/j.foodres.2013.08.020
Di Giovacchino, L., Sestili, S., & Di Vincenzo, D. (2002). Influence of olive processing on virgin olive oil quality. European Journal of Lipid Science and Technology, 104(9–10), 587–601.
EFSA Panel on Dietetic Products and Allergies. (2011). Scientific opinion on the substantiation of health claims related to polyphenols in olive oil and protection of LDL particles from oxidative damage. http://www.efsa.europa.eu/en/efsajournal/pub/2033. Accessed 12 September 2020
Fortini, M., Migliorini, M., Cherubini, C., Cecchi, L., Guerrini, L., Masella, P., & Parenti, A. (2016). Shelf life and quality of olive oil filtered without vertical centrifugation. European Journal of Lipid Science and Technology, 118, 1213–1222.
Fortini, M., Migliorini, M., Cherubini, C., Cecchi, L., & Calamai, L. (2017). Multiple internal standard normalization for improving HS-SPME-GC-MS quantitation in virgin olive oil volatile organic compounds (VOO-VOCs) profile. Talanta, 165, 641–652. https://doi.org/10.1016/j.talanta.2016.12.082
Fregapane, G., Lavelli, V., León, S., Kapuralin, J., & Desamparados Salvador, M. (2006). Effect of filtration on virgin olive oil stability during storage. European Journal of Lipid Science and Technology, 108, 134–142.
Fregapane, G., & Salvador, M. D. (2013). Production of superior quality extra virgin olive oil modulating the content and profile of its minor components. Food Research International, 54(2), 1907–1914. https://doi.org/10.1016/j.foodres.2013.04.022
Funes, E., Allouche, Y., Beltrán, G., Aguliera, M. P., & Jiménez, A. (2018). Predictive ANN models for the optimization of extra virgin olive oil clarification by means of vertical centrifugation. Journal of Food Process Engineering, 41(1), e12593. https://doi.org/10.1111/JFPE.12593
García, J. M., Yousfi, K., Mateos, R., Olmo, M., & Cert, A. (2001). Reduction of oil bitterness by heating of olive (Olea europaea) Fruits. Journal of Agricultural and Food Chemistry, 49(9), 4231–4235. https://doi.org/10.1021/jf001302n
Genovese, A., Mondola, F., Paduano, A., & Sacchi, R. (2020). Biophenolic compounds influence the in-mouth perceived intensity of virgin olive oil flavours and off-flavours. Molecules, 25(8), 1969. https://doi.org/10.3390/molecules25081969
Guerrini, L., Breschi, C., Zanoni, B., Calamai, L., Angeloni, G., Masella, P., & Parenti, A. (2020a). Filtration scheduling: Quality changes in freshly produced virgin olive oil. Foods, 9(8), 1067.
Guerrini, L., Masella, P., Angeloni, G., Migliorini, M., & Parenti, A. (2017). Changes in olive paste composition during decanter feeding and effects on oil yield. European Journal of Lipid Science and Technology, 119(12), 1700223. https://doi.org/10.1002/ejlt.201700223
Guerrini, L., Masella, P., Angeloni, G., & Parenti, A. (2018). Stripping of dissolved oxygen from extra virgin olive oil: Effects on oxidation and biophenols. Journal of Food Processing and Preservation, 42(12), e13832. https://doi.org/10.1111/jfpp.13832
Guerrini, L., Zanoni, B., Breschi, C., Angeloni, G., Masella, P., Calamai, L., & Parenti, A. (2020b). Understanding olive oil stability using filtration and high hydrostatic pressure. Molecules, 25(2), 420. https://doi.org/10.3390/molecules25020420
Hamilton, R. J., & Allen, J. C. (1994). Rancidity in foods. New York: Springer.
Inarejos-García, A. M., Fregapane, G., & Salvador, M. D. (2011). Effect of crushing on olive paste and virgin olive oil minor components. European Food Research and Technology, 232(3), 441–451. https://doi.org/10.1007/s00217-010-1406-4
International Olive Council, 2017., p. COI/T.20/Doc No 29/, 1.
Kalua, C. M., Allen, M. S., Bedgood, D. R., Jr., Bishop, A. G., Prenzler, P. D., & Robards, K. (2007). Olive oil volatile compounds, flavour development and quality: A critical review. Food Chemistry, 100(1), 273–286.
Lozano-Sánchez, J., Cerretani, L., Bendini, A., Segura-Carretero, A., & Fernández-Gutiérrez, A. (2010). Filtration process of extra virgin olive oil: Effect on minor components, oxidative stability and sensorial and physicochemical characteristics. Trends in Food Science and Technology, 21(4), 201–211. https://doi.org/10.1016/j.tifs.2009.12.004
Mannheim, C. H., & Soffer, T. (1996). Shelf-life extension of cottage cheese by modified atmosphere packaging. LWT-Food Science and Technology, 29(8), 767–771.
Masella, P., Parenti, A., Calamai, L., & Spugnoli, P. (2009). Influence of Vertical Centrifugation on Extra Virgin Olive Oil Quality. Journal of the American Oil Chemists’ Society, 86(11), 1137. https://doi.org/10.1007/s11746-009-1445-9
Masella, P., Parenti, A., Spugnoli, P., & Calamai, L. (2011). Malaxation of olive paste under sealed conditions. Journal of the American Oil Chemists’ Society, 88(6), 871–875. https://doi.org/10.1007/s11746-010-1739-y
Migliorini, M., Mugelli, M., Cherubini, C., Viti, P., & Zanoni, B. (2006). Influence of O2 on the quality of virgin olive oil during malaxation. Journal of the Science of Food and Agriculture, 86(13), 2140–2146. https://doi.org/10.1002/JSFA.2588
Morales, M. T., Luna, G., & Aparicio, R. (2005). Comparative study of virgin olive oil sensory defects. Food Chemistry, 91, 293–330.
Morrone, L., Pupillo, S., Neri, L., Bertazza, G., Magli, M., & Rotondi, A. (2017). Influence of olive ripening degree and crusher typology on chemical and sensory characteristics of Correggiolo virgin olive oil. Journal of the Science of Food and Agriculture, 97(5), 1443–1450. https://doi.org/10.1002/jsfa.7883
Pérez-Jiménez, F., Ruano, J., Perez-Martinez, P., Lopez-Segura, F., & Lopez-Miranda, J. (2007). The influence of olive oil on human health: Not a question of fat alone. Molecular Nutrition & Food Research, 51(10), 1199–1208.
Polari, J. J., Garcí-Aguirre, D., Olmo-García, L., Carrasco-Pancorbo, A., & Wang, S. C. (2018). Impact of industrial hammer mill rotor speed on extraction efficiency and quality of extra virgin olive oil. Food Chemistry, 242, 362–368. https://doi.org/10.1016/j.foodchem.2017.09.003
Rodis, P. S., Karathanos, V. T., & Mantzavinou, A. (2002). Partitioning of olive oil antioxidants between oil and water phases. Journal of Agricultural and Food Chemistry, 50(3), 596–601. https://doi.org/10.1021/jf010864j
Servili, M., Sordini, B., Esposto, S., Urbani, S., Veneziani, G., Di Maio, I., et al. (2014). Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants, 3(1), 1–23. https://doi.org/10.3390/antiox3010001
Silva, F. A., Borges, F., & Ferreira, M. A. (2001). Effects of phenolic propyl esters on the oxidative stability of refined sunflower oil. Journal of Agricultural and Food Chemistry, 49(8), 3936.
Tamborrino, A., Pati, S., Romaniello, R., Quinto, M., Zagaria, R., & Leone, A. (2014). Design and implementation of an automatically controlled malaxer pilot plant equipped with an in-line oxygen injection system into the olive paste. Journal of Food Engineering, 141, 1–12. https://doi.org/10.1016/j.jfoodeng.2014.05.002
Tovar, M., Motilva, M. J., & Romero, M. P. (2001). Changes in the phenolic composition of virgin olive oil from young trees (Olea europaea L. cv. Arbequina) grown under linear irrigation strategies. Journal of Agricultural and Food Chemistry, 49(11), 5502–5508. https://doi.org/10.1021/jf0102416
Trapani, S., Breschi, C., Cecchi, L., Guerrini, L., Mulinacci, N., Parenti, A., et al. (2017). Indirect indices of oxidative damage to phenolic compounds for the implementation of olive paste malaxation optimization charts. Journal of Food Engineering, 207, 24–34. https://doi.org/10.1016/j.jfoodeng.2017.03.012
Uceda, M., & Frias, L. (1975). Harvest dates. Evolution of the fruit oil content, oil composition and oil quality. Proceedings of the II Seminario Oleícola Internacional (pp. 125–128). Cordoba: International Olive Oil Council.
Veneziani, G., Novelli, E., Esposto, S., Taticchi, A., & Servili, M. (2017). Applications of recovered bioactive compounds in food products. In C. M. Galanakis (Ed.), Olive Mill Waste: Recent Advances for Sustainable Management (pp. 231–253). Cambridge: Academic Press. https://doi.org/10.1016/B978-0-12-805314-0.00011-X
Vierhuis, E., Servili, M., Baldioli, M., Schols, H. A., Voragen, A. G. J., & Montedoro, G. (2001). Effect of Enzyme Treatment during Mechanical Extraction of Olive Oil on Phenolic Compounds and Polysaccharides. Journal of Agricultural and Food Chemistry, 49(3), 1218–1223. https://doi.org/10.1021/jf000578s
Yorulmaz, A., Tekin, A., & Turan, S. (2011). Improving olive oil quality with double protection: Destoning and malaxation in nitrogen atmosphere. European Journal of Lipid Science and Technology, 113(5), 637–643. https://doi.org/10.1002/ejlt.201000481
Acknowledgements
The authors would like to thank Leonardo Francalanci, Director of the San Michele in Torri Farm Agricultural Society, Scandicci (FI) Italy, for hosting the trials, and David Bagnoli for technical support.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Angeloni Giulia: conceptualization, formal analysis, investigation, data curation, writing–original draft, writing–review and editing. Agnese Spadi: conceptualization, methodology, formal analysis, investigation, data curation, writing–original draft, review and editing. Ferdinando Corti: formal analysis, investigation, data curation. Lorenzo Guerrini: investigation. Luca Calamai: methodology, data curation. Alessandro Parenti: conceptualization, resources, supervision, review and editing. Piernicola Masella: conceptualization, resources, writing–review and editing, project administration, data curation.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Angeloni, G., Spadi, A., Corti, F. et al. Investigation of the Effectiveness of a Vertical Centrifugation System Coupled with an Inert Gas Dosing Device to Produce Extra Virgin Olive Oil. Food Bioprocess Technol 15, 2456–2467 (2022). https://doi.org/10.1007/s11947-022-02884-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02884-3