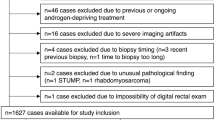
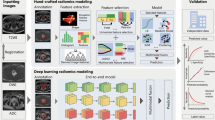
Abstract
Purpose of Review
Benign prostatic hyperplasia (BPH) is prevalent in nearly 70% of men over the age of 60, leading to significant clinical challenges due to varying symptom presentations and treatment responses. The decision to undergo surgical intervention is not straightforward; the American Urological Association recommends consideration of surgical treatment after inadequate or failed response to medical therapy. This review explores the role of artificial intelligence (AI), including machine learning and deep learning models, in enhancing the decision-making processes for BPH management.
Recent Findings
AI applications in this space include analysis of non-invasive imaging modalities, such as multiparametric Magnetic Resonance Imaging (MRI) and Ultrasound, which enhance diagnostic precision. AI models also concatenate serum biomarkers and histopathological analysis to distinguish BPH from prostate cancer (PC), offering high accuracy rates. Furthermore, AI aids in predicting patient outcomes post-treatment, supporting personalized medicine, and optimizing therapeutic strategies.
Summary
AI has demonstrated potential in differentiating BPH from PC through advanced imaging and predictive models, improving diagnostic accuracy, and reducing the need for invasive procedures. Despite promising advancements, challenges remain in integrating AI into clinical workflows, establishing standard evaluation metrics, and achieving cost-effectiveness. Here, we underscore the potential of AI to improve patient outcomes, streamline BPH management, and reduce healthcare costs, especially with continued research and development in this transformative field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data Availability
No datasets were generated or analysed during the current study.
References
Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005;173(4):1256–61.
D’Agate S, et al. Model-based meta-analysis of individual international prostate Symptom score trajectories in patients with benign prostatic hyperplasia with moderate or severe symptoms. Br J Clin Pharmacol. 2020;86(8):1585–99.
Sandhu JS, et al. Management of lower urinary tract symptoms attributed to Benign Prostatic Hyperplasia (BPH): AUA Guideline Amendment 2023. J Urol. 2024;211(1):11–9.
Winograd J, et al. Emerging drugs for the treatment of benign prostatic hyperplasia: a 2023 update. Expert Opin Emerg Drugs; 2024.
Winograd J, et al. Search trends in treatment for benign prostatic hyperplasia: a twenty-year analysis. Asian Journal of Urology; 2023.
Sandhu JS, Dahm BB. Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia (BPH): AUA Guideline amendment 2023. J Urol. 2023. https://doi.org/10.1097/JU.0000000000003698.
Derevianko A et al. The Use of Artificial Intelligence (AI) in the Radiology Field: what is the state of doctor-patient communication in Cancer diagnosis? Cancers (Basel), 2023. 15(2).
Ahn JS, et al. Artificial intelligence in breast Cancer diagnosis and Personalized Medicine. J Breast Cancer. 2023;26(5):405–35.
Luengo-Fernandez R, et al. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14(12):1165–74.
Khanna NN et al. Economics of Artificial Intelligence in Healthcare: diagnosis vs. treatment. Healthc (Basel), 2022. 10(12).
Brawer MK. Prostate-specific antigen: current status. CA Cancer J Clin. 1999;49(5):264–81.
Mochtar CA, et al. PSA velocity in conservatively managed BPH: can it predict the need for BPH-related invasive therapy? Prostate. 2006;66(13):1407–12.
Merrick GS, et al. Prostate-specific antigen (PSA) velocity and benign prostate hypertrophy predict for PSA spikes following prostate brachytherapy. Brachytherapy. 2003;2(3):181–8.
Mehralivand S, et al. A cascaded deep learning-based Artificial Intelligence Algorithm for Automated Lesion detection and classification on biparametric prostate magnetic resonance imaging. Acad Radiol. 2022;29(8):1159–68.
Bermejo P, et al. Development of interpretable predictive models for BPH and prostate Cancer. Clin Med Insights Oncol. 2015;9:15–24.
Megherbi DB, Soper B. Effect of feature selection on machine learning algorithms for more accurate predictor of surgical outcomes in Benign Pro Static Hyperplasia cases (BPH). in 2011 IEEE International Conference on Computational Intelligence for Measurement Systems and Applications (CIMSA) Proceedings. 2011.
Shah M, et al. Artificial intelligence (AI) in urology-current use and future directions: an iTRUE study. Turk J Urol. 2020;46(1):S27–39.
Checcucci E, et al. Artificial intelligence and neural networks in urology: current clinical applications. Minerva Urol Nefrol. 2020;72(1):49–57.
Stam WT, et al. The prediction of surgical complications using artificial intelligence in patients undergoing major abdominal surgery: a systematic review. Surgery. 2022;171(4):1014–21.
Fu J, Ye J, Cui W. The dice measure of cubic hesitant fuzzy sets and its initial evaluation method of benign prostatic hyperplasia symptoms. Sci Rep. 2019;9(1):60.
Torshizi AD, et al. A hybrid fuzzy-ontology based intelligent system to determine level of severity and treatment recommendation for Benign Prostatic Hyperplasia. Comput Methods Programs Biomed. 2014;113(1):301–13.
Fu J, Ye J. Simplified Neutrosophic Exponential Similarity measures for the initial Evaluation/Diagnosis of Benign Prostatic Hyperplasia Symptoms. Symmetry. 2017;9(8):154.
Lu Q, et al. Identifying Benign Prostatic Hyperplasia stages by measuring the length of the Proximal Prostatic Urethra: An Operator-Error-Free early-screening Ultrasonography Method with a uniquely-calibrated standardized plane. IEEE Access. 2019;7:185908–15.
Tzelves L, et al. Cluster Analysis Assessment in proposing a Surgical technique for Benign Prostatic Enlargement. Stud Health Technol Inf. 2022;295:466–9.
Mourmouris P, et al. The use and applicability of machine learning algorithms in predicting the surgical outcome for patients with benign prostatic enlargement. Which model to use? Arch Ital Urol Androl. 2021;93(4):418–24.
T JMC et al. Automated classification of significant prostate Cancer on MRI: a systematic review on the performance of machine learning applications. Cancers (Basel), 2020. 12(6).
Nieboer D, van der Ploeg T, Steyerberg EW. Assessing discriminative performance at External Validation of Clinical Prediction models. PLoS ONE. 2016;11(2):e0148820.
Choo MS, et al. Development of decision support formulas for the prediction of bladder outlet obstruction and prostatic surgery in patients with lower urinary tract Symptom/Benign Prostatic Hyperplasia: part I, Development of the Formula and its internal validation. Int Neurourol J. 2017;21(Suppl 1):S55–65.
Jung E, et al. Enhancement of Perivascular spaces using densely connected deep convolutional neural network. IEEE Access. 2019;7:18382–91.
Kong H, et al. Evaluation of an Analytic Reconstruction Method as a platform for spectral cone-beam CT. IEEE Access. 2018;6:21314–23.
Berlin, Clara et al. “Novel AI-Based Algorithm for the Automated Computation of Coronal Parameters in Adolescent Idiopathic Scoliosis Patients: A Validation Study on 100 Preoperative Full Spine X-Rays.” Global spine journal 2024;14(6):1728–1737. https://doi.org/10.1177/21925682231154543
Huang TL, et al. Transfer learning with CNNs for efficient prostate cancer and BPH detection in transrectal ultrasound images. Sci Rep. 2023;13(1):21849.
Liu J, et al. Deep convolutional neural networks for Raman spectrum recognition: a unified solution. Analyst. 2017;142(21):4067–74.
Acquarelli J, et al. Convolutional neural networks for vibrational spectroscopic data analysis. Anal Chim Acta. 2017;954:22–31.
Zhang Q, et al. Multimodal feature learning and fusion on B-mode ultrasonography and sonoelastography using point-wise gated deep networks for prostate cancer diagnosis. Biomed Tech (Berl). 2020;65(1):87–98.
Huang X et al. Texture Feature-Based Classification on Transrectal Ultrasound Image for Prostatic Cancer Detection. Comput Math Methods Med, 2020. 2020: p. 7359375.
Imani F, et al. Computer-aided prostate Cancer detection using Ultrasound RF Time Series: in vivo feasibility study. IEEE Trans Med Imaging. 2015;34(11):2248–57.
Mehmood M, et al. A classifier model for prostate cancer diagnosis using CNNs and transfer learning with multi-parametric MRI. Front Oncol. 2023;13:1225490.
Khosravi P, et al. A Deep Learning Approach to Diagnostic classification of prostate Cancer using Pathology-Radiology Fusion. J Magn Reson Imaging. 2021;54(2):462–71.
Zhou B et al. Learning Deep Features for Discriminative Localization. in. 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). 2016.
Cary KC, Cooperberg MR. Biomarkers in prostate cancer surveillance and screening: past, present, and future. Ther Adv Urol. 2013;5(6):318–29.
Liu YF, et al. Radiomics-Based Machine Learning models for Predicting P504s/P63 immunohistochemical expression: a Noninvasive Diagnostic Tool for prostate Cancer. Front Oncol. 2022;12:911426.
Wibmer A, et al. Haralick texture analysis of prostate MRI: utility for differentiating non-cancerous prostate from prostate cancer and differentiating prostate cancers with different Gleason scores. Eur Radiol. 2015;25(10):2840–50.
Khalid SU, Syed A, Shah SSH. Machine learning approaches for the histopathological diagnosis of prostatic hyperplasia. Volume 11. ANNALS OF CLINICAL AND ANALYTICAL MEDICINE; 2020. pp. 425–8. 5.
Yoneyama T, et al. Characteristics of alpha2,3-sialyl N-glycosylated PSA as a biomarker for clinically significant prostate cancer in men with elevated PSA level. Prostate. 2021;81(16):1411–27.
Matsuzaki J, Ochiya T. Circulating microRNAs: next-generation Cancer detection. Keio J Med. 2020;69(4):88–96.
Urabe F, et al. Large-scale circulating microRNA profiling for the liquid biopsy of prostate Cancer. Clin Cancer Res. 2019;25(10):3016–25.
Ramirez-Garrastacho M, et al. Extracellular vesicles as a source of prostate cancer biomarkers in liquid biopsies: a decade of research. Br J Cancer. 2022;126(3):331–50.
Narita T, et al. Clinical implications of serum N-glycan profiling as a diagnostic and prognostic biomarker in germ-cell tumors. Cancer Med. 2017;6(4):739–48.
Ishikawa T et al. An Automated Micro-total Immunoassay System for Measuring Cancer-Associated alpha2,3-linked sialyl N-Glycan-carrying prostate-specific Antigen May improve the accuracy of prostate Cancer diagnosis. Int J Mol Sci, 2017. 18(2).
Matsumoto T, et al. Serum N-glycan profiling is a potential biomarker for castration-resistant prostate cancer. Sci Rep. 2019;9(1):16761.
Iwamura H, et al. Machine learning diagnosis by immunoglobulin N-glycan signatures for precision diagnosis of urological diseases. Cancer Sci. 2022;113(7):2434–45.
Kodama H, et al. N-glycan signature of serum immunoglobulins as a diagnostic biomarker of urothelial carcinomas. Cancer Med. 2021;10(4):1297–313.
Tanaka T et al. Aberrant N-Glycosylation Profile of serum immunoglobulins is a diagnostic biomarker of Urothelial Carcinomas. Int J Mol Sci, 2017. 18(12).
Kumar D, et al. Metabolomics-derived prostate cancer biomarkers: fact or fiction? J Proteome Res. 2015;14(3):1455–64.
Mondul AM, et al. Metabolomic analysis of prostate cancer risk in a prospective cohort: the alpha-tocolpherol, beta-carotene cancer prevention (ATBC) study. Int J Cancer. 2015;137(9):2124–32.
Wang Y, et al. Multimodal convolutional neural networks based on the Raman spectra of serum and clinical features for the early diagnosis of prostate cancer. Spectrochim Acta Mol Biomol Spectrosc. 2023;293:122426.
Etzioni R, Cha R, Cowen ME. Serial prostate specific antigen screening for prostate cancer: a computer model evaluates competing strategies. J Urol. 1999;162(3 Pt 1):741–8.
Nagendran M, et al. Artificial intelligence versus clinicians: systematic review of design, reporting standards, and claims of deep learning studies. BMJ. 2020;368:m689.
Bansal S, et al. Applications of artificial intelligence in benign prostatic hyperplasia. Artif Intell Surg. 2023;3(2):129–39.
Funding
There was no funding for this review.
Author information
Authors and Affiliations
Contributions
J.L. and J.W. wrote the main manuscript text and prepared Table 1. All authors helped with conceptualization, methodology, and manuscript review.
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors were performed in accordance with all applicable ethical standards including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lama, J., Winograd, J., Codelia-Anjum, A. et al. AI for BPH Surgical Decision-Making: Cost Effectiveness and Outcomes. Curr Urol Rep 26, 4 (2025). https://doi.org/10.1007/s11934-024-01240-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s11934-024-01240-6