Abstract
Rheumatoid arthritis (RA) is an autoimmune and chronic inflammatory disease that causes joint deformation. Till now several studies has been carried out promising its cure, but curing has not yet achieved to the satisfactory levels. Herbal approach to treat disease by a cross-kingdom mechanism via exogenous miRNA is an emerging trend to target associated genes with RA pathogenesis as a therapeutic potential. The concept of acquired/exogenous miRNA into pathophysiological prospect provides an opportunity to explore inter-species kingdom like regulation of plant miRNAs on human health. The change in gene expression was attributed by a short 22-24 nucleotide long sequence that binds to its complementary region to suppress/silence the gene expression. This makes exogenous miRNA a novel approach for targeted therapy to treat complex chronic inflammatory diseases. Here, aim of the review was to address significance of plant derived miRNA based targeted therapy to regulate inflammation in RA.
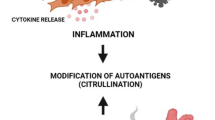
Graphical abstract


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis. Nat Rev Dis Pri. 2018;4:18001. This article describes the heterogeneity of RA disease.
World Health Organization. Available from ttps://www.who.int/chp/topics/rheumatic/en/. Accessed 2019.
Gierut A, Perlman H, Pope RM. Innate immunity and rheumatoid arthritis. Rheum Dis Clin. 2010;36(2):271–96. https://doi.org/10.1016/j.rdc.2010.03.004.
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15. https://doi.org/10.1038/s41413-018-0016-9.
Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105.
Dharap A, Pokrzywa C, Murali S, Pandi G, Vemuganti R. MicroRNA miR-324-3p induces promoter-mediated expression of RelA gene. PLoS One. 2013;8(11):e79467. https://doi.org/10.1371/journal.pone.0079467.
Marín RM, Sulc M, Vanícek J. Searching the coding region for microRNA targets. RNA. 2013;19(4):467–74. https://doi.org/10.1261/rna.035634.112.
Brümmer A, Hausser J. MicroRNA binding sites in the coding region of mRNAs: extending the repertoire of post-transcriptional gene regulation. Bioessays. 2014;36(6):617–26. https://doi.org/10.1002/bies.201300104.
Chandan K, Gupta M, Sarwat M. Role of Host and Pathogen-Derived MicroRNAs in Immune Regulation During Infectious and Inflammatory Diseases. Front Immunol. 24(10):3081. https://doi.org/10.3389/fimmu.2019.03081. This article describes the role of plant exogenous miRNA in alteration of host gene expression.
Sanchita T. R, Asif MH, Trivedi PK. Dietary plant miRNAs as an augmented therapy: cross-kingdom gene regulation. RNA Biol. 2018;15(12):1433–9. https://doi.org/10.1080/15476286.2018.1551693. This article describes the importance of dietary miRNA in cross-kingdom regulation.
Li Z, Xu R, Li N. MicroRNAs from plants to animals, do they define a new messenger for communication? Nutr Metab. 2018;15:68. https://doi.org/10.1186/s12986-018-0305-8. This article describes the conservation of miRNA between plants and animals.
Lukasik A, Brzozowska I, Zielenkiewicz U, Zielenkiewicz P. Detection of Plant miRNAs Abundance in Human Breast Milk. Int J Mol Sci. 2017;19(1):37. https://doi.org/10.3390/ijms19010037. This article describes the presence of plant based miRNA in Human body fluid.
Wang W, Liu D, Zhang X, Chen D, Cheng Y, Shen F. Plant MicroRNAs in Cross-Kingdom Regulation of Gene Expression. Int J Mol Sci. 2018;19(7):2007. https://doi.org/10.3390/ijms19072007. This article describes the use of exogenous miRNA as a therapeutic target to treat various diseases.
Ardekani AM, Naeini MM. The Role of MicroRNAs in Human Diseases. Avicenna J Med Biotechnol. 2010;2(4):161–79.
Liang H, Huang L, Cao J, Zen K, Chen X, Zhang CY. Regulation of mammalian gene expression by exogenous microRNAs. Wiley Interdiscip Rev RNA. 2012;3(5):733–42. https://doi.org/10.1002/wrna.1127.
Chamnanchanunt S, Kuroki C, Desakorn V, Enomoto M, Thanachartwet V, Sahassananda D, et al. Downregulation of plasma miR-451 and miR-16 in Plasmodium vivax infection. Exp Parasitol. 2015;155:19–25. https://doi.org/10.1016/j.exppara.2015.04.013.
Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific. Micro RNA Sci. 2005;309(5740):1577–81. https://doi.org/10.1126/science.1113329.
Kitab B, Alj HS, Ezzikouri S, Benjelloun S. MicroRNAs as Important Players in Host-hepatitis B Virus Interactions. J Clin Transl Hepatol. 2015;3(2):149–61. https://doi.org/10.14218/JCTH.2015.00002.
Moran-Moguel MC, Petarra-Del Rio S, Mayorquin-Galvan EE, Zavala-Cerna MG. Rheumatoid Arthritis and miRNAs: A Critical Review through a Functional View. J Immunol Res. 2018;2018:2474529–16. https://doi.org/10.1155/2018/2474529. This article describes the functional differences between plant and animal miRNA.
Catalanotto C, Cogoni C, Zardo G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int J Mol Sci. 2016;17(10):1712. https://doi.org/10.3390/ijms17101712.
Zeng J, Gupta VK, Jiang Y, Yang B, Gong L, Zhu H. Cross-Kingdom Small RNAs Among Animals. Plants Microbes Cells. 2019;8(4):371. https://doi.org/10.3390/cells8040371. This article describes the way of communication through conserved exogenous miRNA among kingdoms.
Fang Y, Spector DL. Identification of nuclear dicing bodies containing proteins for microRNA biogenesis in living Arabidopsis plants. Curr Biol. 2007;17:818–23. https://doi.org/10.1016/j.cub.2007.04.005.
Song L, Han MH, Lesicka J, Fedoroff N. Arabidopsis primary microRNA processing proteins HYL1 and DCL1 define a nuclear body distinct from the Cajal body. PNA Sci USA. 2007;104:5437–42. https://doi.org/10.1073/pnas.0701061104.
Xie Z, Allen E, Fahlgren N, Calamar A, Givan SA, Carrington JC. Expression of Arabidopsis MIRNA genes. Plant Physiol. 2005;138:2145–54. https://doi.org/10.1104/pp.105.062943.
Kurihara Y, Watanabe Y. Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. PNA Sci USA. 2004;101:12753–8. https://doi.org/10.1073/pnas.0403115101.
Yu B, Yang Z, Li J, Minakhina S, Yang M, Padgett RW, et al. Methylation as a crucial step in plant microRNA biogenesis. Sci. 2005;307:932–5. https://doi.org/10.1126/science.1107130.
Huang Y, Ji L, Huang Q, Vassylyev DG, Chen X, Ma JB. Structural insights into mechanisms of the small RNA methyltransferase HEN1. Nat. 2009;461(7265):823–7. https://doi.org/10.1038/nature08433.
Yu B, Yang Z, Li J, Minakhina S, Yang M, Padgett RW, et al. Methylation as a crucial step in plant microRNA biogenesis. Sci. 2005;307(5711):932–5. https://doi.org/10.1126/science.1107130.
Park MY, Wu G, Gonzalez-Sulser A, Vaucheret H, Poethig RS. Nuclear processing and export of microRNAs in Arabidopsis. PNA Sci USA. 2005;102:3691–6. https://doi.org/10.1073/pnas.0405570102.
Borchert GM, Lanier W, Davidson BL. RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol. 2006;13:1097–101. https://doi.org/10.1038/nsmb1167.
Ruby JG, Stark A, Johnston WK, Kellis M, Bartel DP, Lai EC. Evolution, biogenesis, expression, and target predictions of a substantially expanded set of Drosophila microRNAs. Genome Res. 2007;17:1850–64. https://doi.org/10.1101/gr.6597907.
Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12(2):99–110. https://doi.org/10.1038/nrg2936.
Ipsaro JJ, Joshua-Tor L. From guide to target: molecular insights into eukaryotic RNA-interference machinery. Nat Struct Mol. 2015;22(1):20–8. https://doi.org/10.1038/nsmb.2931.
Forman JJ, Legesse-Miller A, Coller HA. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. PNA Sci USA. 2008;105(39):14879–84. https://doi.org/10.1073/pnas.0803230105.
Zhang J, Zhou W, Liu Y, Liu T, Li C, Wang L. Oncogenic role of microRNA-532-5p in human colorectal cancer via targeting of the 5'UTR of RUNX3. Oncol Lett. 2018;15(5):7215–20. https://doi.org/10.3892/ol.2018.8217. This article describes miRNA which can bind complementary region within 5’UTR.
Dharap A, Pokrzywa C, Murali S, Pandi G, Vemuganti R. MicroRNA miR-324-3p induces promoter-mediated expression of RelA gene. PLoS One. 2013;8(11):e79467. https://doi.org/10.1371/journal.pone.0079467.
Jo MH, Shin S, Jung SR, Kim E, Song JJ, Hohng S. Human Argonaute 2 Has Diverse Reaction Pathways on Target RNAs. Mol Cell. 2015;59(1):117–24. https://doi.org/10.1016/j.molcel.2015.04.027.
Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet. 2015;16(7):421–33. https://doi.org/10.1038/nrg3965.
Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3:e3694.
Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51.
Li Z, Xu R, Li N. MicroRNAs from plants to animals, do they define a new messenger for communication? Nutr Metab. 2018;15:68.
Kinet V, Halkein J, Dirkx E, Windt LJ. Cardiovascular extracellular microRNAs: emerging diagnostic markers and mechanisms of cell-to-cell RNA communication. Front Genet. 2013;4:214. https://doi.org/10.3389/fgene.2013.00214.
Ji L, Chen X. Regulation of small RNA stability: methylation and beyond. Cell Res. 2012;22:624–36.
Liang H, Zen K, Zhang J, Zhang CY, Chen X. New roles for microRNAs in cross-species communication. RNA Biol. 2013;10:367–70.
Zhou Z, Li X, Liu J, Dong L, Chen Q, Liu J, et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res. 2015;25:39–49.
Zhang L, Hou D, Chen X, Li D, Zhu L, Zhang Y, et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res. 2012;22:107–26.
Hou D, He F, Ma L, Cao M, Zhou Z, Wei Z, et al. The potential atheroprotective role of plant MIR156a as a repressor of monocyte recruitment on inflamed human endothelial cells. J Nutr Biochem. 2018;57:197–205. This article describes the inhibitory role of green vegetables derived miR156a in human aortic endothelial cells.
Liang G, Zhu Y, Sun B, Shao Y, Jing A, Wang J, et al. Assessing the survival of exogenous plant microRNA in mice. Food Sci Nutr. 2014;2:380–8.
Chen X, Dai GH, Ren ZM, Tong YL, Yang F, Zhu YQ. Identification of dietetically absorbed rapeseed (Brassica campestris L.) bee pollen microRNAs in serum of mice. Biomed Res Int. 2016;2016:1–5.
Chin AR, Fong MY, Somlo G, Wu J, Swiderski P, Wu X, et al. Cross-kingdom inhibition of breast cancer growth by plant miR159. Cell Res. 2016;26:217–28.
Li J, Zhang Y, Li D, Liu Y, Chu D, Jiang X, et al. Small non-coding RNAs transfer through mammalian placenta and directly regulate fetal gene expression. Protein Cell. 2015;6(6):391–6. https://doi.org/10.1007/s13238-015-0156-2.
Zhou Z, Li X, Liu J, Dong L, Chen Q, Liu J, et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res. 2015;25:39–49.
Li Z, Xu R, Li N. MicroRNAs from plants to animals, do they define a new messenger for communication? Nutri Metab. 2018;15:68. https://doi.org/10.1186/s12986-018-0305-8.
Liu YC, Chen WL, Kung WH, Huang HD. Plant miRNAs found in human circulating system provide evidences of cross kingdom RNAi. BMC Genomics. 2017;18:12.
Kumar D, Kumar S, Ayachit G, Bhairappanavar SB, Ansari A, Sharma P, Soni S, Das J. Cross-kingdom regulation of putative miRNAs derived from happy tree in cancer pathway: a systems biology approach. Int J Mol Sci. 2017;18(6):1191. https://doi.org/10.3390/ijms18061191. This article describes the role of medicinal plant derived miRNA in Cancer.
Sharma A, Sahu S, Kumari P, Gopi SR, Malhotra R, Biswas S. Genome-wide identification and functional annotation of miRNAs in anti-inflammatory plant and their cross-kingdom regulation in homo sapiens. J Biomol Struct Dyn. 2017;35:1389–400. This article describes plant miRNA, as a therapeutic approach in RA.
LaMonte G, Philip N, Reardon J, Lacsina JR, Majoros W, Chapman L, et al. Translocation of sickle cell erythrocyte microRNAs into Plasmodium falciparum inhibits parasite translation and contributes to malaria resistance. Cell Host Microbe. 2012;12:187–99.
Samols MA, Skalsky RL, Maldonado AM, Riva A, Lopez MC, Baker HV, et al. Identification of cellular genes targeted by KSHV-encoded microRNAs. PLoS Pathog. 2007;3:e65.
Jopling CL, Norman KL, Sarnow P. Positive and negative modulation of viral and cellular mRNAs by liver-specific microRNA miR-122. Cold Spring Harb Symp Quant Biol. 2006;71:369–76. https://doi.org/10.1101/sqb.2006.71.022.
Ledda B, Ottaggio L, Izzotti A, Sukkar SG, Miele M. Small RNAs in eucaryotes: new clues for amplifying microRNA benefits. Cell Bioscience. 2020;10:1. https://doi.org/10.1186/s13578-019-0370-3.
Condrat CE, Thompson DC, Barbu MG, Bugnar OL, Boboc A, Cretoiu D, et al. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells. 2020;9(2):276. https://doi.org/10.3390/cells9020276. This article describes circulatory miRNA as disease specific biomarker.
Baumann V, Winkler J. miRNA-based therapies: strategies and delivery platforms for oligonucleotide and non-oligonucleotide agents. Future Med Chem. 2014;6(17):1967–84. https://doi.org/10.4155/fmc.14.116.
Bader AG. miR-34 - a microRNA replacement therapy is headed to the clinic. Front Genet. 2012;3:120. https://doi.org/10.3389/fgene.2012.00120.
Zhang Y, Yun Z, Gong L, Qu H, Duan X, Jiang Y, et al. Comparison of miRNA Evolution and Function in Plants and Animals. Microrna. 2018;7(1):4–10. https://doi.org/10.2174/2211536607666180126163031.
Acknowledgement
We acknowledge Council of Scientific and Industrial Research (CSIR), Project code MLP 2013 and Department of Science and Technology (DST), Government of India, New Delhi, India for providing financial support. Mohd Saquib received fellowship support from CSIR, Prachi Agnihotri received fellowship from DST project GAP0212, and Monu received fellowship support from CSIR. We also thank Council of Scientific and Industrial Research (CSIR)-Institute of Genomics and Integrative Biology, Delhi, India for research and AcSIR for academic support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declared no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Rheumatoid Arthritis
Key message
• Functional implication of plant miRNAs in human is a useful strategy in therapeutic aspect.
• Plant exogenous miRNA and host mRNA interactions might have a potential role in the alteration of genetic regulation of host cellular machinery.
• The ability of plant miRNA to regulate the cross-kingdom mRNA translation can be used for the treatment of RA.
Rights and permissions
About this article
Cite this article
Saquib, M., Agnihotri, P., Monu et al. Exogenous miRNA: A Perspective Role as Therapeutic in Rheumatoid Arthritis. Curr Rheumatol Rep 23, 43 (2021). https://doi.org/10.1007/s11926-021-01009-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s11926-021-01009-7