Abstract
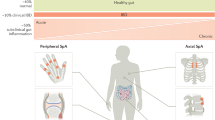
Spondyloarthropathies (SpA) are a group of related disorders with common clinical and genetic characteristics. The prototype disease in this group is ankylosing spondylitis; other entities include reactive arthritis, psoriatic arthritis, and arthritis in patients with inflammatory bowel disease. Over recent years, there has been a special interest in the relation between spondylitis/ synovitis and gut inflammation in patients with SpA. Two thirds of patients with undifferentiated SpA show histologic signs of gut inflammation, and a fraction of these patients go on to develop clinically overt Crohn’s disease. In this review, the authors will focus on 1) the growing evidence that has been provided that gut inflammation in SpA is immunologically related to Crohn’s disease, based on the molecular characterization of the inflammation (lymphocyte homing markers and ligands, T cell cytokines, macrophage markers, and serology); and 2) on the therapeutic implications resulting from this concept. The recent introduction and positioning of anti-tumor necrosis factoralpha therapy in patients with ankylosing spondylitis and other types of SpA is, in large part, based on this concept.
Similar content being viewed by others
References and Recommended Reading
Wright V: Seronegative polyarthritis: a unified concept. Arthritis Rheum 1978, 21:619–633.
De Keyser F, Elewaut D, De Vos M, et al.: Bowel inflammation and the spondyloarthropathies. Rheum Dis Clin North Am 1998, 24:785–813. Exhaustive review on the relation between gut and joint inflammation in patients with SpA, summarizing especially clinical and pathologic data.
Mielants H, Veys EM, Cuvelier C, De Vos M: Ileocolonoscopic findings in seronegative spondylarthropathies. Brit J Rheumatol 1988, 27(suppl):95–105.
Mielants H, Veys EM: The gut in the spondyloarthropathies. J Rheumatol 1990, 17:7–10.
Simenon G, Van Gossum A, Adler M, et al.: Macroscopic and microscopic gut lesions in seronegative spondyloarthropathies. J Rheumatol 1990, 17:1491–1494.
Leirisalo-Repo M, Turunen U, Stenman S, et al.: High frequency of silent inflammatory bowel disease in spondylarthropathy. Arthritis Rheum 1994, 37:23–31.
Altomonte I, Zoli A, Veneziani A, et al.: Clinically silent inflammatory gut lesions in undifferentiated spondyloarthropathies. Clin Rheumatol 1994, 13:565–570.
Mielants H, Veys EM, Cuvelier C, et al.: Gut inflammation in children with late onset pauciarticular juvenile chronic arthritis and evolution to adult spondyloarthropathy-a prospective study. J Rheumatol 1993, 20:1567–1572.
Schatteman L, Mielants H, Veys EM, et al.: Gut inflammation in psoriasis arthritis: a prospective ileocolonoscopic study. J Rheumatol 1995, 22:680–683.
Cuvelier C, Barbatis C, Mielants H, et al.: Histopathology of intestinal inflammation related to reactive arthritis. Gut 1987, 28:394–401.
Mielants H, Veys EM, Joos R, et al.: Repeat ileocolonoscopy in reactive arthritis. J Rheumatol 1987, 14:456–458.
Mielants H, Veys EM, Cuvelier C, et al.: The evolution of spondylarthropathies in relation to gut histology. Part II: histological aspects. J Rheumatol 1995, 22:2273–2278.
Mielants H, Veys EM, Cuvelier C, et al.: The evolution of spondylarthropathies in relation to gut histology. Part III: relation between gut and joint. J Rheumatol 1995, 22:2279–2284.
Gravallese EM, Kantrowitz FG: Arthritic manifestations of inflammatory bowel disease. Am J Gastroenterol 1988, 83:703–709.
Orchard TR, Wordsworth BP, Jewell DP: Peripheral arthro pathies in inflammatory bowel disease: their articular distribution and natural history. Gut 1998, 42:387–391. Extensive retrospective survey of the prevalence of arthritis in patients with Crohn’s disease and ulcerative colitis at the Oxford Inflammatory Bowel Disease Clinic.
de Vlam K, De Vos M, Mielants H, et al.: Spondyloarthropathy is underestimated in inflammatory bowel disease: prevalence and HLA association. J Rheumatol 2000, 27:2860–2865. Cross-sectional study of the prevalence of SpA and SpA-related signs and symptoms in a cohort of patients with inflammatory bowel disease at the Ghent University Hospital.
Palm O, Moum B, Jahnsen J, Gran JT: The prevalence and incidence of peripheral arthritis in patients with inflammatory bowel disease, a prospective population-based study (the IBSEN study). Rheumatology 2001, 40:1256–1261. Study of the occurrence of peripheral arthritis in a population-based cohort of inflammatory bowel disease patients.
Palm O, Moum B, Ongre A, Gran JT: Prevalence of ankylosing spondylitis and other spondyloarthropathies among patients with inflammatory bowel disease: a population study (the IBSEN study). J Rheumatol 2002, 29:511–515.
Mielants H, Veys EM, Cuvelier C, et al.: The evolution of spondylarthropathies in relation to gut histology. Part I: clinical aspects. J Rheumatol 1995, 22:2266–2272.
Mielants H, Veys EM, Goethals K, et al.: Destructive hip lesions in seronegative spondylarthropathies: relation to gut inflammation. J Rheumatol 1990, 17:335–340.
Helliwell PS, Hickling P, Wricht V: Do the radiological changes of classic ankylosing spondylitis differ from the changes found in the spondylitis associated with inflammatory bowel disease, psoriasis and reactive arthritis? Ann Rheum Dis 1998, 57:135–140.
Van Damme N, Elewaut D, Baeten D, et al.: Gut mucosal T cell lines from ankylosing spondylitis patients are enriched with alphaEbeta7 integrin. Clin Exp Rheumatol 2001, 19:681–687.
Elewaut D, De Keyser F, Cuvelier C, et al.: Distinctive activated cellular subsets in colon from patients with Crohn’s disease and ulcerative colitis. Scand J Gastroenterol 1998, 33:743–748.
Demetter P, De Vos M, Van Damme N, et al.: Focal upregulation of E-cadherin-catenin complex in inflamed bowel mucosa but reduced expression in ulcer-associated lineage. Am J Clin Pathol 2000, 114:364–370.
Demetter P, Baeten D, De Keyser F, et al.: Subclinical gut inflammation in spondyloarthropathy patients is associated with upregulation of the E-cadherin/catenin complex. Ann Rheum Dis 2000, 59:211–216.
Baeten D, Demetter P, Cuvelier C, et al.: Macrophages expressing the scavenger receptor CD163: a link between immune alterations of the gut and synovial inflammation in spondyloarthropathy. J Pathol 2002, 196:343–350. Identification of an interesting macrophage population, which is selectively increased in SpA synovium, as well as in gut mucosa of patients with SpA and Crohn’s disease.
Cope AP, Londei M, Chu NR, et al.: Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J Clin Invest 1994, 94:749–760.
Van Damme HN, De Keyser F, Demetter P, et al.: The proportion of Th1 cells, which prevail in gut mucosa, is decreased in inflammatory bowel syndrome. Clin Exp Immunol 2001, 125:383–390.
Van Damme N, De Vos M, Baeten D, et al.: Flow cytometric analysis of gut mucosal lymphocytes supports an impaired Th1 cytokine profile in spondyloarthropathy. Ann Rheum Dis 2001, 60:495–499.
Peeters M, Joossens S, Vermeire S, et al.: Diagnostic value of anti-Saccharomyces serevisiae and antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease. Am J Gastroenterol 2001, 96:730–734.
Vermeire S, Joossens S, Peeters M, et al.: Comparative study of ASCA (Anti-Saccharomyces cerevisiae antibody) assays in inflammatory bowel disease. Gastroenterology 2001, 120:827–833.
Hoffman I, Veys EM, Peeters H, et al.:Antisaccharomyces cerevisiae antibodies in ankylosing spondylitis. Arthritis Rheum 2001, 44(suppl):S236.
De Keyser F, Van Damme N, De Vos M, et al.: Opportunities for immune modulation in the spondyloarthropathies with special reference to gut inflammation. Inflamm Res 2000, 49:47–54.
van Dullemen HM, Van Deventer SJ, Hommes DW, et al.: Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology 1995, 109:129–135.
D’haens G, Van Deventer S, Van Hogezand R, et al.: Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn’s disease: A European multicenter trial. Gastroenterology 1999, 116:1029–1034.
Present DH, Rutgeerts P, Targan S, et al.: Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999, 340:1398–1405.
Hanauer SB, Feagan BG, Lichtenstein GR, et al.: Maintenance infliximab for Crohn’s disease: the ACCENT I randomized trial. Lancet 2002, 359:1541–1549.
Sandborn WJ, Feagan BG, Hanauer SB, et al.: An engineered human antibody to TNF (CDP571) for active Crohn’s disease: a randomized double-blind placebo-controlled trial. Gastroenterology 2001, 120:1330–1338.
Van Deventer SJ, Elson CO, Fedorak RN: Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn’s disease. Crohn’s Disease Study Group. Gastroenterology 1997, 113:383–389.
Fedorak RN, Gangl A, Elson CO, et al.: Recombinant interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology 2000, 119:1473–1482.
Schreiber S, Fedorak RN, Nielsen OH, et al.: Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Crohn’s Disease IL-10 Cooperative Study Group. Gastroenterology 2000, 119:1461–1472.
Colombel JF, Rutgeerts P, Malchow H, et al.: Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn’s disease. Gut 2001, 49:42–46.
Steidler L, Hans W, Schotte L, et al.: Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 2000, 289:1352–1355.
Sands BE, Bank S, Sninsky CA, et al.: Preliminary evaluation of safety and activity of recombinant human interleukin 11 in patients with active Crohn’s disease. Gastroenterology 1999, 117:58–64.
Bernstein CN, Sargent M, Gallatin WM: Beta2 integrin/ICAM expression in Crohn’s disease. Clin Immunol Immunopathol 1998, 86:147–160.
Kirwan I, Nielsen OH: LFA-1 subunit expression in ulcerative colitis patients. Dig Dis Sci 1996, 41:670–676.
Bennett CF, Condon T, Grimm S, et al.: Inhibition of endothelial cell-leukocyte adhesion molecule expression with antisense oligonucleotides. J Immunol 1994, 152:3530–3540.
Yacyshyn BR, Bowen-Yacyshyn MB, Jewell L, et al.: A placebocontrolled trial of ICAM-1 antisense oligonucleotide in the treatment of Crohn’s disease. Gastroenterology 1998, 114:1133–1142.
Podolsky K, Lobb R, King N, et al.: Attenuation of colitis in the cotton-top tamarin by anti-a4 integrin monoclonal antibody. J Clin Invest 1993, 92:372–380.
Hesterberg PE, Winsor-Hine D, Briskin MJ, et al.: Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin alpha4beta7. Gastroenterology 1996, 111:1373–1380.
Van den Bosch F, Kruithof E, De Vos M, et al.: Crohn’s disease associated with spondyloarthropathy: effect of TNF-a blockade with infliximab on the articular symptoms. Lancet 2000, 356:1821–1822. First indication of effectiveness of anti-TNFγ therapy on locomotor SpA-related manifestations in patients with active (gut and joints) Crohn’s disease.
Van den Bosch F, Kruithof E, Baeten D, et al.: Effects of a loading dose regimen of 3 infusions of chimeric monoclonal antibody to tumour necrosis factor a (infliximab) in spondyloarthropathy: an open pilot study. Ann Rheum Dis 2000, 59:428–433. This open pilot study in patients with different types of SpA and active disease strongly suggests effectivity of infliximab therapy on axial and peripheral locomotor manifestations, as well as on biologic inflammatory parameters.
Kruithof E, Van den Bosch F, Baeten D, et al.: Repeated infusions of infliximab, a chimeric anti-TNFa monoclonal antibody, in patients with active spondyloarthropathy: one-year follow- up. Ann Rheum Dis 2002, 61:207–212.
Brandt J, Haibel H, Cornely D, et al.: Successful treatment of active ankylosing spondylitis with the anti-tumor necrosis factor a monoclonal antibody infliximab. Arthritis Rheum 2000, 43:1346–1352. This open pilot study in patients with active ankylosing spondylitis strongly suggests effectivity of infliximab therapy.
Brandt J, Haibel H, Reddig J, et al.: J Successful short term treatment of severe undifferentiated spondyloarthropathy with the anti-tumor necrosis factor-alpha monoclonal antibody infliximab. J Rheumatol 2002, 29:118–122.
Van den Bosch F, Kruithof E, Baeten D, et al.: Randomized double-blind comparison of chimeric monoclonal antibody to tumor necrosis factor a (infliximab) versus placebo in active spondyloarthropathy. Arthritis Rheum 2002, 46:755–765. Controlled study of infliximab therapy in patients with active SpA, demonstrating significant efficacy.
Braun J, Brandt J, Listing J, et al.: Treatment of active ankylosing spondylitis with infliximab: randomised controlled multicenter trial. Lancet 2002, 359:1187–1193. Controlled study of infliximab therapy in patients with active ankylosing spondylitis, demonstrating significant efficacy.
Gorman JD, Sack KE, Davis JC: Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor a. N Engl J Med 2002, 346:1349–1356. Controlled study of etanercept therapy in patients with active ankylosing spondylitis, demonstrating significant efficacy.
Breban M, Gombert B, Amor B, Dougados M: Efficacy of thalidomide in the treatment of refractory ankylosing spondylitis. Arthritis Rheum 1999, 42:580–581.
Breban M, Dougados M: Reply to the letter by Lee et al. Arthritis Rheum 2001, 44:2457–2458.
Lee L, Lawford R, McNeil HP: The efficacy of thalidomide in severe refractory seronegative spondylarthropathy. Arthritis Rheum 2001, 44:2456–2457.
De Rycke L, Van Damme N, Kruithof E, et al.: Induction of ANA, anti-dsDNA and anti-nucleosome antibodies following infliximab treatment in rheumatoid arthritis and spondyloarthropathy patients. Arthritis Res 2002, 4:A28-A29.
Sandborn WJ, Hanauer SB, Katz S, et al.: Etanercept for active Crohn’s disease: a randomized, double-blind, placebocontrolled trial. Gastroenterology 2001, 121:1088–1094.
Mease PJ, Goffe BS, Metz J, et al.: Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 2000, 356:385–390.
Chaudhari U, Romano P, Mulcahy LD, et al.: Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomized trial. Lancet 2001, 357:1842–1847.
Kruithof E, Kestelyn P, Elewaut D, et al.: Successful use of infliximab in a patient with treatment resistant spondyloarthropathy related uveitis. Ann Rheum Dis 2002, 61:470.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
De Keyser, F., Baeten, D., Van den Bosch, F. et al. Gut inflammation and spondyloarthropathies. Curr Rheumatol Rep 4, 525–532 (2002). https://doi.org/10.1007/s11926-002-0061-6
Issue Date:
DOI: https://doi.org/10.1007/s11926-002-0061-6