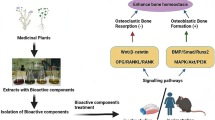
Abstract
Purpose of Review
This review aims to provide a theoretical basis and insights for quercetin’s clinical application in the prevention and treatment of osteoporosis (OP), analyzing its roles in bone formation promotion, bone resorption inhibition, anti-inflammation, antioxidant effects, and potential mechanisms.
Recent Findings
OP, a prevalent bone disorder, is marked by reduced bone mineral density and impaired bone architecture, elevating the risk of fractures in patients. The primary approach to OP management is pharmacotherapy, with quercetin, a phytochemical compound, emerging as a focus of recent interest. This natural flavonoid exerts regulatory effects on bone marrow mesenchymal stem cells, osteoblasts, and osteoclasts and promotes bone health and metabolic equilibrium via anti-inflammatory and antioxidative pathways.
Summary
Although quercetin has demonstrated significant potential in regulating bone metabolism, there is a need for further high-quality clinical studies focused on medicinal quercetin.

Similar content being viewed by others
Data Availability
No datasets were generated or analyzed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Föger-Samwald U, Dovjak P, Azizi-Semrad U, Kerschan-Schindl K, Pietschmann P. Osteoporosis: pathophysiology and therapeutic options. EXCLI J. 2020;19:1017–37. https://doi.org/10.17179/excli2020-2591.
Johnston CB, Dagar M. Osteoporosis in older adults. Med Clin North Am. 2020;104(5):873–84. https://doi.org/10.1016/j.mcna.2020.06.004.
Liu P, Wang W, Li Z, Li Y, Yu X, Tu J, et al. Ferroptosis: a new regulatory mechanism in osteoporosis. Oxid Med Cell Longev. 2022;2022:2634431. https://doi.org/10.1155/2022/2634431.
Imamudeen N, Basheer A, Iqbal AM, Manjila N, Haroon NN, Manjila S. Management of osteoporosis and spinal fractures: contemporary guidelines and evolving paradigms. Clin Med Res. 2022;20(2):95–106. https://doi.org/10.3121/cmr.2021.1612.
Langdahl BL. Overview of treatment approaches to osteoporosis. Br J Pharmacol. 2021;178(9):1891–906. https://doi.org/10.1111/bph.15024.
Strampel W, Emkey R, Civitelli R. Safety considerations with bisphosphonates for the treatment of osteoporosis. Drug Saf. 2007;30(9):755–63. https://doi.org/10.2165/00002018-200730090-00003.
Park SY, Kim SH, Kim TY, Lee YK, Ha YC, Jang S, et al. Incidence and risk of venous thromboembolism in bisphosphonates and selective estrogen receptor modulators treatment in Korea. J Korean Med Sci. 2021;36(27):e186. https://doi.org/10.3346/jkms.2021.36.e186.
Everts-Graber J, Lehmann D, Burkard JP, Schaller B, Gahl B, Häuselmann H, et al. Risk of osteonecrosis of the jaw under denosumab compared to bisphosphonates in patients with osteoporosis. J Bone Miner Res. 2022;37(2):340–8. https://doi.org/10.1002/jbmr.4472.
Sleeman A, Clements JN. Abaloparatide: a new pharmacological option for osteoporosis. Am J Health Syst Pharm. 2019;76(3):130–5. https://doi.org/10.1093/ajhp/zxy022.
Lim SY. Romosozumab for the treatment of osteoporosis in women: efficacy, safety, and cardiovascular risk. Womens Health (Lond). 2022;18:17455057221125576. https://doi.org/10.1177/17455057221125577.
Georgiou N, Kakava MG, Routsi EA, Petsas E, Stavridis N, Freris C, et al. Quercetin: a potential polydynamic drug. Molecules (Basel). 2023;28(24):8141. https://doi.org/10.3390/molecules28248141.
Gong J, Pan X, Zhou X, Zhu F. Dietary quercetin protects Cherax quadricarinatus against white spot syndrome virus infection. J Invertebr Pathol. 2023;198:107931. https://doi.org/10.1016/j.jip.2023.107931.
Wang G, Wang Y, Yao L, Gu W, Zhao S, Shen Z, et al. Pharmacological activity of quercetin: an updated review. Evid Based Complement Alternat Med. 2022;2022:3997190. https://doi.org/10.1155/2022/3997190.
Zhang W, Zheng Y, Yan F, Dong M, Ren Y. Research progress of quercetin in cardiovascular disease. Front Cardiovasc Med. 2023;10:1203713. https://doi.org/10.3389/fcvm.2023.1203713.
Shi GJ, Li Y, Cao QH, Wu HX, Tang XY, Gao XH, et al. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: a systematic review of the literature. Biomed Pharmacother. 2019;109:1085–99. https://doi.org/10.1016/j.biopha.2018.10.130.
•• Cao L, Wang J, Zhang Y, Tian F, Wang C. Osteoprotective effects of flavonoids: evidence from in vivo and in vitro studies (Review). Mol Med Rep. 2022;25(6):200. https://doi.org/10.3892/mmr.2022.12716. This paper highlights the potential of quercetin as a natural therapeutic agent in the prevention and treatment of bone-related diseases by summarizing the results of published experimental and clinical studies.
Salvio G, Ciarloni A, Gianfelice C, Lacchè F, Sabatelli S, Giacchetti G, et al. The effects of polyphenols on bone metabolism in postmenopausal women: systematic review and meta-analysis of randomized control trials. Antioxidants (Basel). 2023;12(10):1830. https://doi.org/10.3390/antiox12101830.
Baş A, Albeniz I. Investigation of the effects of eugenol and quercetin on bone loss in STZ-NA induced diabetic rats utilizing micro CT. J Diabetes Metab Disord. 2022;21(1):637–46. https://doi.org/10.1007/s40200-022-01026-y.
Sun J, Pan Y, Li X, Wang L, Liu M, Tu P, et al. Quercetin attenuates osteoporosis in orchiectomy mice by regulating glucose and lipid metabolism via the GPRC6A/AMPK/mTOR signaling pathway. Front Endocrinol. 2022;13:849544. https://doi.org/10.3389/fendo.2022.849544.
Ge YW, Feng K, Liu XL, Zhu ZA, Chen HF, Chang YY, et al. Quercetin inhibits macrophage polarization through the p-38α/β signalling pathway and regulates OPG/RANKL balance in a mouse skull model. J Cell Mol Med. 2020;24(5):3203–16. https://doi.org/10.1111/jcmm.14995.
Xiong Y, Huang CW, Shi C, Peng L, Cheng YT, Hong W, et al. Quercetin suppresses ovariectomy-induced osteoporosis in rat mandibles by regulating autophagy and the NLRP3 pathway. Exp Biol Med (Maywood). 2023;248(23):2363–80. https://doi.org/10.1177/15353702231211977.
Pandit AP, Omase SB, Mute VM. A chitosan film containing quercetin-loaded transfersomes for treatment of secondary osteoporosis. Drug Deliv Transl Res. 2020;10(5):1495–506. https://doi.org/10.1007/s13346-020-00708-5.
Zhou Y, Wu Y, Ma W, Jiang X, Takemra A, Uemura M, et al. The effect of quercetin delivery system on osteogenesis and angiogenesis under osteoporotic conditions. J Mater Chem B. 2017;5(3):612–25. https://doi.org/10.1039/c6tb02312f.
Abd El-Fattah AI, Fathy MM, Ali ZY, El-Garawany AEA, Mohamed EK. Enhanced therapeutic benefit of quercetin-loaded phytosome nanoparticles in ovariectomized rats. Chem Biol Interact. 2017;271:30–8. https://doi.org/10.1016/j.cbi.2017.04.026.
•• Wang Y, Che L, Chen X, He Z, Song D, Yuan Y, et al. Repurpose dasatinib and quercetin: targeting senescent cells ameliorates postmenopausal osteoporosis and rejuvenates bone regeneration. Bioact Mater. 2023;25:13–28. https://doi.org/10.1016/j.bioactmat.2023.01.009. Quercetin targets senescent BMSCs, restores the function of senescent BMSCs, improves skeletal homeostasis, and effectively exerts its anti-OP effects.
Feng L, Yang Z, Hou N, Wang M, Lu X, Li Y, et al. Long non-coding RNA Malat1 increases the rescuing effect of quercetin on TNFα-impaired bone marrow stem cell osteogenesis and ovariectomy-induced osteoporosis. Int J Mol Sci. 2023;24(6):5965. https://doi.org/10.3390/ijms24065965.
Mousavi S, Vakili S, Zal F, Savardashtaki A, Jafarinia M, Sabetian S, et al. Quercetin potentiates the anti-osteoporotic effects of alendronate through modulation of autophagy and apoptosis mechanisms in ovariectomy-induced bone loss rat model. Mol Biol Rep. 2023;50(4):3693–703. https://doi.org/10.1007/s11033-023-08311-w.
Vakili S, Zal F, Mostafavi-Pour Z, Savardashtaki A, Koohpeyma F. Quercetin and vitamin E alleviate ovariectomy-induced osteoporosis by modulating autophagy and apoptosis in rat bone cells. J Cell Physiol. 2021;236(5):3495–509. https://doi.org/10.1002/jcp.30087.
Xing LZ, Ni HJ, Wang YL. Quercitrin attenuates osteoporosis in ovariectomized rats by regulating mitogen-activated protein kinase (MAPK) signaling pathways. Biomed Pharmacother. 2017;89:1136–41. https://doi.org/10.1016/j.biopha.2017.02.073.
Gao Q, Wang L, Wang S, Huang B, Jing Y, Su J. Bone marrow mesenchymal stromal cells: identification, classification, and differentiation. Front Cell Dev Biol. 2021;9:787118. https://doi.org/10.3389/fcell.2021.787118.
Ning K, Liu S, Yang B, Wang R, Man G, Wang DE, et al. Update on the effects of energy metabolism in bone marrow mesenchymal stem cells differentiation. Mol Metab. 2022;58:101450. https://doi.org/10.1016/j.molmet.2022.101450.
Kurenkova AD, Medvedeva EV, Newton PT, Chagin AS. Niches for skeletal stem cells of mesenchymal origin. Front Cell Dev Biol. 2020;8:592. https://doi.org/10.3389/fcell.2020.00592.
Qadir A, Liang S, Wu Z, Chen Z, Hu L, Qian A. Senile osteoporosis: the involvement of differentiation and senescence of bone marrow stromal cells. Int J Mol Sci. 2020;21(1):349. https://doi.org/10.3390/ijms21010349.
Zhang Q, Liang N, He B, Wu S, Wen D, Tang X, et al. SDF-1 induces directional chemotaxis of BMSCs at the intervertebral fusion site and promotes osteogenic differentiation by regulating Wnt/β-catenin in the bone marrow chimera spinal intervertebral fusion mouse model. Turk J Biol. 2023;47(1):14–28. https://doi.org/10.55730/1300-0152.2638.
Han Y, Yang J, Fang J, Zhou Y, Candi E, Wang J, et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct Target Ther. 2022;7(1):92. https://doi.org/10.1038/s41392-022-00932-0.
Wang L, Dai HW, Zheng J, Zhou J, Chen DS. Effect of quercetin on the cell cycle and adhesion molecules of NOD/SCID mice with acute B lymphocytic leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2018;26(6):1616–20. https://doi.org/10.7534/j.issn.1009-2137.2018.06.006.
Ramesh P, Jagadeesan R, Sekaran S, Dhanasekaran A, Vimalraj S. Flavonoids: classification, function, and molecular mechanisms involved in bone remodelling. Front Endocrinol. 2021;12:779638. https://doi.org/10.3389/fendo.2021.779638.
Li CJ, Cheng P, Liang MK, Chen YS, Lu Q, Wang JY, et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Investig. 2015;125(4):1509–22. https://doi.org/10.1172/jci77716.
Wang X, Zou X, Zhao J, Wu X, E L, Feng L, et al. Site-specific characteristics of bone marrow mesenchymal stromal cells modify the effect of aging on the skeleton. Rejuvenation Res. 2016;19(5):351–61. https://doi.org/10.1089/rej.2015.1766
Xing X, Tang Q, Zou J, Huang H, Yang J, Gao X, et al. Bone-targeted delivery of senolytics to eliminate senescent cells increases bone formation in senile osteoporosis. Acta Biomater. 2023;157:352–66. https://doi.org/10.1016/j.actbio.2022.11.056.
Xing X, Huang H, Gao X, Yang J, Tang Q, Xu X, et al. Local elimination of senescent cells promotes bone defect repair during aging. ACS Appl Mater Interfaces. 2022;14(3):3885–99. https://doi.org/10.1021/acsami.1c22138.
Khotib J, Marhaeny HD, Miatmoko A, Budiatin AS, Ardianto C, Rahmadi M, et al. Differentiation of osteoblasts: the links between essential transcription factors. J Biomol Struct Dyn. 2023;41(19):10257–76. https://doi.org/10.1080/07391102.2022.2148749.
Ren M, Wang X, Hu M, Jiang Y, Xu D, Xiang H, et al. Enhanced bone formation in rat critical-size tibia defect by a novel quercetin-containing alpha-calcium sulphate hemihydrate/nano-hydroxyapatite composite. Biomed Pharmacother. 2022;146:112570. https://doi.org/10.1016/j.biopha.2021.112570.
Schlesinger PH, Blair HC, Beer Stolz D, Riazanski V, Ray EC, Tourkova IL, et al. Cellular and extracellular matrix of bone, with principles of synthesis and dependency of mineral deposition on cell membrane transport. Am J Physiol Cell Physiol. 2020;318(1):C111–24. https://doi.org/10.1152/ajpcell.00120.2019.
Muñoz A, Docaj A, Ugarteburu M, Carriero A. Poor bone matrix quality: what can be done about it? Curr Osteoporos Rep. 2021;19(5):510–31. https://doi.org/10.1007/s11914-021-00696-6.
Wang X, Schröder HC, Feng Q, Diehl-Seifert B, Grebenjuk VA, Müller WE. Isoquercitrin and polyphosphate co-enhance mineralization of human osteoblast-like SaOS-2 cells via separate activation of two RUNX2 cofactors AFT6 and Ets1. Biochem Pharmacol. 2014;89(3):413–21. https://doi.org/10.1016/j.bcp.2014.03.020.
Raj Preeth D, Saravanan S, Shairam M, Selvakumar N, Selestin Raja I, Dhanasekaran A, et al. Bioactive Zinc(II) complex incorporated PCL/gelatin electrospun nanofiber enhanced bone tissue regeneration. Eur J Pharm Sci. 2021;160:105768. https://doi.org/10.1016/j.ejps.2021.105768.
Vimalraj S, Rajalakshmi S, Raj Preeth D, Vinoth Kumar S, Deepak T, Gopinath V, et al. Mixed-ligand copper(II) complex of quercetin regulate osteogenesis and angiogenesis. Mater Sci Eng C Mater Biol Appl. 2018;83:187–94. https://doi.org/10.1016/j.msec.2017.09.005.
• Vlashi R, Zhang X, Wu M, Chen G. Wnt signaling: essential roles in osteoblast differentiation, bone metabolism and therapeutic implications for bone and skeletal disorders. Genes Dis. 2023;10(4):1291–317. https://doi.org/10.1016/j.gendis.2022.07.011. The important role of the WNT signaling pathway in bone metabolism is elucidated, as well as its interactions with other signaling pathways in the potential gene regulatory network of bone metabolism.
Littman J, Yang W, Olansen J, Phornphutkul C, Aaron RK. LRP5, Bone mass polymorphisms and skeletal disorders. Genes (Basel). 2023;14(10):1846. https://doi.org/10.3390/genes14101846.
Predes D, Maia LA, Matias I, Araujo HPM, Soares C, Barros-Aragão FGQ, et al. The flavonol quercitrin hinders GSK3 activity and potentiates the Wnt/β-catenin signaling pathway. Int J Mol Sci. 2022;23(20):12078. https://doi.org/10.3390/ijms232012078.
Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem. 2006;99(5):1233–9. https://doi.org/10.1002/jcb.20958.
Komori T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem Cell Biol. 2018;149(4):313–23. https://doi.org/10.1007/s00418-018-1640-6.
He X, Liu W, Liu Y, Zhang K, Sun Y, Lei P, et al. Nano artificial periosteum PLGA/MgO/Quercetin accelerates repair of bone defects through promoting osteogenic - angiogenic coupling effect via Wnt/ β-catenin pathway. Materials Today Bio. 2022;16:100348. https://doi.org/10.1016/j.mtbio.2022.100348.
Bian W, Xiao S, Yang L, Chen J, Deng S. Quercetin promotes bone marrow mesenchymal stem cell proliferation and osteogenic differentiation through the H19/miR-625-5p axis to activate the Wnt/β-catenin pathway. BMC Complement Med Ther. 2021;21(1):243. https://doi.org/10.1186/s12906-021-03418-8.
Koosha E, Eames BF. Two Modulators of Skeletal Development: BMPs and proteoglycans. J Dev Biol. 2022;10(2):15. https://doi.org/10.3390/jdb10020015.
Huse K, Bakkebø M, Wälchli S, Oksvold MP, Hilden VI, Forfang L, et al. Role of Smad proteins in resistance to BMP-induced growth inhibition in B-cell lymphoma. PLoS ONE. 2012;7(10):e46117. https://doi.org/10.1371/journal.pone.0046117.
Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233–41. https://doi.org/10.1080/08977190412331279890.
Biswas S, Li P, Wu H, Shafiquzzaman M, Murakami S, Schneider MD, et al. BMPRIA is required for osteogenic differentiation and RANKL expression in adult bone marrow mesenchymal stromal cells. Sci Rep. 2018;8(1):8475. https://doi.org/10.1038/s41598-018-26820-8.
Zhang J, Liu Z, Luo Y, Li X, Huang G, Chen H, et al. The role of flavonoids in the osteogenic differentiation of mesenchymal stem cells. Front Pharmacol. 2022;13:849513. https://doi.org/10.3389/fphar.2022.849513.
Pang XG, Cong Y, Bao NR, Li YG, Zhao JN. Quercetin stimulates bone marrow mesenchymal stem cell differentiation through an estrogen receptor-mediated pathway. Biomed Res Int. 2018;2018:4178021. https://doi.org/10.1155/2018/4178021.
• Li X, Chen R, Lei X, Wang P, Zhu X, Zhang R, et al. Quercetin regulates ERα mediated differentiation of BMSCs through circular RNA. Gene. 2021;769:145172. https://doi.org/10.1016/j.gene.2020.145172. This study elucidates one of the molecular mechanisms through which quercetin exerts its phytoestrogen-like effect to promote osteogenic differentiation of BMSCs, potentially via the miR-326-5p-mRNA regulatory network.
Hayrapetyan A, Jansen JA, van den Beucken JJ. Signaling pathways involved in osteogenesis and their application for bone regenerative medicine. Tissue Eng Part B Rev. 2015;21(1):75–87. https://doi.org/10.1089/ten.TEB.2014.0119.
Suzuki A, Guicheux J, Palmer G, Miura Y, Oiso Y, Bonjour JP, et al. Evidence for a role of p38 MAP kinase in expression of alkaline phosphatase during osteoblastic cell differentiation. Bone. 2002;30(1):91–8. https://doi.org/10.1016/s8756-3282(01)00660-3.
Matsuguchi T, Chiba N, Bandow K, Kakimoto K, Masuda A, Ohnishi T. JNK activity is essential for Atf4 expression and late-stage osteoblast differentiation. J Bone Miner Res. 2009;24(3):398–410. https://doi.org/10.1359/jbmr.081107.
Xiao G, Jiang D, Thomas P, Benson MD, Guan K, Karsenty G, et al. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem. 2000;275(6):4453–9. https://doi.org/10.1074/jbc.275.6.4453.
Greenblatt MB, Shim JH, Zou W, Sitara D, Schweitzer M, Hu D, et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J Clin Investig. 2010;120(7):2457–73. https://doi.org/10.1172/jci42285.
Zhang Q, Chang B, Zheng G, Du S, Li X. Quercetin stimulates osteogenic differentiation of bone marrow stromal cells through miRNA-206/connexin 43 pathway. Am J Transl Res. 2020;12(5):2062–70.
Zhou Y, Wu Y, Jiang X, Zhang X, Xia L, Lin K, et al. The effect of quercetin on the osteogenesic differentiation and angiogenic factor expression of bone marrow-derived mesenchymal stem cells. PLoS ONE. 2015;10(6):e0129605. https://doi.org/10.1371/journal.pone.0129605.
Li Y, Wang J, Chen G, Feng S, Wang P, Zhu X, et al. Quercetin promotes the osteogenic differentiation of rat mesenchymal stem cells via mitogen-activated protein kinase signaling. Exp Ther Med. 2015;9(6):2072–80. https://doi.org/10.3892/etm.2015.2388.
Prouillet C, Mazière JC, Mazière C, Wattel A, Brazier M, Kamel S. Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblasts through ERK and estrogen receptor pathway. Biochem Pharmacol. 2004;67(7):1307–13. https://doi.org/10.1016/j.bcp.2003.11.009.
Yahara Y, Nguyen T, Ishikawa K, Kamei K, Alman BA. The origins and roles of osteoclasts in bone development, homeostasis and repair. Development.. 2022;149(8):dev199908. https://doi.org/10.1242/dev.199908.
Woo JT, Nakagawa H, Notoya M, Yonezawa T, Udagawa N, Lee IS, et al. Quercetin suppresses bone resorption by inhibiting the differentiation and activation of osteoclasts. Biol Pharm Bull. 2004;27(4):504–9. https://doi.org/10.1248/bpb.27.504.
Córdoba A, Manzanaro-Moreno N, Colom C, Rønold HJ, Lyngstadaas SP, Monjo M, et al. Quercitrin nanocoated implant surfaces reduce osteoclast activity in vitro and in vivo. Int J Mol Sci. 2018;19(11):3319. https://doi.org/10.3390/ijms19113319.
Masuhara M, Tsukahara T, Tomita K, Furukawa M, Miyawaki S, Sato T. A relation between osteoclastogenesis inhibition and membrane-type estrogen receptor GPR30. Biochem Biophys Rep. 2016;8:389–94. https://doi.org/10.1016/j.bbrep.2016.10.013.
Kim JM, Lin C, Stavre Z, Greenblatt MB, Shim JH. Osteoblast-osteoclast communication and bone homeostasis. Cells. 2020;9(9):2073. https://doi.org/10.3390/cells9092073.
Jianwei W, Ye T, Hongwei W, Dachuan L, Fei Z, Jianyuan J, et al. The role of TAK1 in RANKL-induced osteoclastogenesis. Calcif Tissue Int. 2022;111(1):1–12. https://doi.org/10.1007/s00223-022-00967-z.
Lee K, Chung YH, Ahn H, Kim H, Rho J, Jeong D. Selective regulation of MAPK signaling mediates RANKL-dependent osteoclast differentiation. Int J Biol Sci. 2016;12(2):235–45. https://doi.org/10.7150/ijbs.13814.
Kim JH, Kim N. Signaling pathways in osteoclast differentiation. Chonnam Med J. 2016;52(1):12–7. https://doi.org/10.4068/cmj.2016.52.1.12.
Shim KS, Ha H, Kim T, Lee CJ, Ma JY. Orostachys japonicus suppresses osteoclast differentiation by inhibiting NFATc1 expression. Am J Chin Med. 2015;43(5):1013–30. https://doi.org/10.1142/s0192415x15500585.
Tsuji M, Yamamoto H, Sato T, Mizuha Y, Kawai Y, Taketani Y, et al. Dietary quercetin inhibits bone loss without effect on the uterus in ovariectomized mice. J Bone Miner Metab. 2009;27(6):673–81. https://doi.org/10.1007/s00774-009-0088-0.
De Leon-Oliva D, Barrena-Blázquez S, Jiménez-Álvarez L, Fraile-Martinez O, García-Montero C, López-González L, et al. The RANK-RANKL-OPG system: a multifaceted regulator of homeostasis, immunity, and cancer. Medicina (Kaunas). 2023;59(10):1752. https://doi.org/10.3390/medicina59101752.
Udagawa N, Koide M, Nakamura M, Nakamichi Y, Yamashita T, Uehara S, et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab. 2021;39(1):19–26. https://doi.org/10.1007/s00774-020-01162-6.
Srivastava S, Bankar R, Roy P. Assessment of the role of flavonoids for inducing osteoblast differentiation in isolated mouse bone marrow derived mesenchymal stem cells. Phytomedicine. 2013;20(8–9):683–90. https://doi.org/10.1016/j.phymed.2013.03.001.
Takito J, Inoue S, Nakamura M. The sealing zone in osteoclasts: a self-organized structure on the bone. Int J Mol Sci. 2018;19(4):984. https://doi.org/10.3390/ijms19040984.
Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40(2):251–64. https://doi.org/10.1016/j.bone.2006.09.023.
Torres HM, Arnold KM, Oviedo M, Westendorf JJ, Weaver SR. Inflammatory processes affecting bone health and repair. Curr Osteoporos Rep. 2023;21(6):842–53. https://doi.org/10.1007/s11914-023-00824-4.
Weitzmann MN. The role of inflammatory cytokines, the RANKL/OPG axis, and the immunoskeletal interface in physiological bone turnover and osteoporosis. Scientifica. 2013;2013:125705. https://doi.org/10.1155/2013/125705.
Marahleh A, Kitaura H, Ohori F, Kishikawa A, Ogawa S, Shen WR, et al. TNF-α directly enhances osteocyte RANKL expression and promotes osteoclast formation. Front Immunol. 2019;10:2925. https://doi.org/10.3389/fimmu.2019.02925.
Ruscitti P, Cipriani P, Carubbi F, Liakouli V, Zazzeroni F, Di Benedetto P, et al. The role of IL-1β in the bone loss during rheumatic diseases. Mediators Inflamm. 2015;2015:782382. https://doi.org/10.1155/2015/782382.
Cui Z, Zhao X, Amevor FK, Du X, Wang Y, Li D, et al. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front Immunol. 2022;13:943321. https://doi.org/10.3389/fimmu.2022.943321.
Ma Z, Zhen Y, Hu C, Yi H. Myeloid-derived suppressor cell-derived arginase-1 oppositely modulates IL-17A and IL-17F through the ESR/STAT3 pathway during colitis in mice. Front Immunol. 2020;11:687. https://doi.org/10.3389/fimmu.2020.00687.
Zhang J, Zhang R, Li W, Ma XC, Qiu F, Sun CP. IκB kinase β (IKKβ): Structure, transduction mechanism, biological function, and discovery of its inhibitors. Int J Biol Sci. 2023;19(13):4181–203. https://doi.org/10.7150/ijbs.85158.
Bhoi A, Dwivedi SD, Singh D, Keshavkant S, Singh MR. Mechanistic prospective and pharmacological attributes of quercetin in attenuation of different types of arthritis. 3 Biotech. 2023;13(11):362. https://doi.org/10.1007/s13205-023-03787-6.
Oh A, Pardo M, Rodriguez A, Yu C, Nguyen L, Liang O, et al. NF-κB signaling in neoplastic transition from epithelial to mesenchymal phenotype. Cell Commun Signal. 2023;21(1):291. https://doi.org/10.1186/s12964-023-01207-z.
Wu Y, Yang Y, Wang L, Chen Y, Han X, Sun L, et al. Effect of bifidobacterium on osteoclasts: TNF-α/NF-κB inflammatory signal pathway-mediated mechanism. Front Endocrinol. 2023;14:1109296. https://doi.org/10.3389/fendo.2023.1109296.
Li Y, Li A, Strait K, Zhang H, Nanes MS, Weitzmann MN. Endogenous TNFalpha lowers maximum peak bone mass and inhibits osteoblastic Smad activation through NF-kappaB. J Bone Miner Res. 2007;22(5):646–55. https://doi.org/10.1359/jbmr.070121.
Jang WY, Hwang JY, Cho JY. Ginsenosides from Panax ginseng as key modulators of NF-κB Signaling Are Powerful Anti-Inflammatory and Anticancer Agents. Int J Mol Sci. 2023;24(7):6119. https://doi.org/10.3390/ijms24076119.
Endale M, Park SC, Kim S, Kim SH, Yang Y, Cho JY, et al. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology. 2013;218(12):1452–67. https://doi.org/10.1016/j.imbio.2013.04.019.
Xiao J, Han Q, Yu Z, Liu M, Sun J, Wu M, et al. Morroniside inhibits inflammatory bone loss through the TRAF6-Mediated NF-κB/MAPK signalling pathway. Pharmaceuticals (Basel). 2023;16(10):1438. https://doi.org/10.3390/ph16101438.
Wang XC, Zhao NJ, Guo C, Chen JT, Song JL, Gao L. Quercetin reversed lipopolysaccharide-induced inhibition of osteoblast differentiation through the mitogen-activated protein kinase pathway in MC3T3-E1 cells. Mol Med Rep. 2014;10(6):3320–6. https://doi.org/10.3892/mmr.2014.2633.
• Zhong R, Miao L, Zhang H, Tan L, Zhao Y, Tu Y, et al. Anti-inflammatory activity of flavonols via inhibiting MAPK and NF-κB signaling pathways in RAW264.7 macrophages. Curr Res Food Sci. 2022;5:1176–84. https://doi.org/10.1016/j.crfs.2022.07.007. Quercetin inhibits the overproduction of nitric oxide, decreases the levels of ROS, TNF-α, and IL-6, and reduces the nuclear translocation of NF-κB p65 to inhibit the activation of NF-κB and MAPK signaling pathways.
• Wang FS, Wu RW, Chen YS, Ko JY, Jahr H, Lian WS. Biophysical modulation of the mitochondrial metabolism and redox in bone homeostasis and osteoporosis: how biophysics converts into bioenergetics. Antioxidants (Basel). 2021;10(9):1394. https://doi.org/10.3390/antiox10091394. This study systematically describes the effects of mitochondrial dysfunction and REDOX system imbalance on bone homeostasis and osteoporosis.
Catheline SE, Kaiser E, Eliseev RA. Mitochondrial genetics and function as determinants of bone phenotype and aging. Curr Osteoporos Rep. 2023;21(5):540–51. https://doi.org/10.1007/s11914-023-00816-4.
Tao H, Ge G, Liang X, Zhang W, Sun H, Li M, et al. ROS signaling cascades: dual regulations for osteoclast and osteoblast. Acta Biochim Biophys Sin. 2020;52(10):1055–62. https://doi.org/10.1093/abbs/gmaa098.
Xu D, Hu MJ, Wang YQ, Cui YL. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules. 2019;24(6):1123. https://doi.org/10.3390/molecules24061123.
Peng J, Yang Z, Li H, Hao B, Cui D, Shang R, et al. Quercetin reprograms immunometabolism of macrophages via the SIRT1/PGC-1α signaling pathway to ameliorate lipopolysaccharide-induced oxidative damage. Int J Mol Sci. 2023;24(6):5542. https://doi.org/10.3390/ijms24065542.
Yan C, Zhang J, An F, Wang J, Shi Y, Yuan L, et al. Research progress of ferroptosis regulatory network and bone remodeling in osteoporosis. Front Public Health. 2022;10:910675. https://doi.org/10.3389/fpubh.2022.910675.
Gao Z, Chen Z, Xiong Z, Liu X. Ferroptosis - a new target of osteoporosis. Exp Gerontol. 2022;165:111836. https://doi.org/10.1016/j.exger.2022.111836.
Jing X, Du T, Chen K, Guo J, Xiang W, Yao X, et al. Icariin protects against iron overload-induced bone loss via suppressing oxidative stress. J Cell Physiol. 2019;234(7):10123–37. https://doi.org/10.1002/jcp.27678.
Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta. 2018;1865(5):721–33. https://doi.org/10.1016/j.bbamcr.2018.02.010.
Yu C, Xiao JH. The Keap1-Nrf2 system: a mediator between oxidative stress and aging. Oxid Med Cell Longev. 2021;2021:6635460. https://doi.org/10.1155/2021/6635460.
Marcucci G, Domazetovic V, Nediani C, Ruzzolini J, Favre C, Brandi ML. Oxidative stress and natural antioxidants in osteoporosis: novel preventive and therapeutic approaches. Antioxidants (Basel). 2023;12(2):373. https://doi.org/10.3390/antiox12020373.
Zhao F, Guo L, Wang X, Zhang Y. Correlation of oxidative stress-related biomarkers with postmenopausal osteoporosis: a systematic review and meta-analysis. Arch Osteoporos. 2021;16(1):4. https://doi.org/10.1007/s11657-020-00854-w.
• Liu X, Tao T, Yao H, Zheng H, Wang F, Gao Y. Mechanism of action of quercetin in rheumatoid arthritis models: meta-analysis and systematic review of animal studies. Inflammopharmacology. 2023;31(4):1629–45. https://doi.org/10.1007/s10787-023-01196-y. A high-quality meta-analysis and systematic review study explored the regulatory role of quercetin in antioxidant indices.
Wang N, Wang L, Yang J, Wang Z, Cheng L. Quercetin promotes osteogenic differentiation and antioxidant responses of mouse bone mesenchymal stem cells through activation of the AMPK/SIRT1 signaling pathway. Phytother Res. 2021;35(5):2639–50. https://doi.org/10.1002/ptr.7010.
Hu G, Yu Y, Sharma D, Pruett-Miller SM, Ren Y, Zhang GF, et al. Glutathione limits RUNX2 oxidation and degradation to regulate bone formation. JCI Insight. 2023;8(16):e166888. https://doi.org/10.1172/jci.insight.166888.
Bardestani A, Ebrahimpour S, Esmaeili A, Esmaeili A. Quercetin attenuates neurotoxicity induced by iron oxide nanoparticles. J Nanobiotechnology. 2021;19(1):327. https://doi.org/10.1186/s12951-021-01059-0.
Xiao J, Zhang G, Chen B, He Q, Mai J, Chen W, et al. Quercetin protects against iron overload-induced osteoporosis through activating the Nrf2/HO-1 pathway. Life Sci. 2023;322:121326. https://doi.org/10.1016/j.lfs.2022.121326.
Lan D, Qi S, Yao C, Li X, Liu H, Wang D, et al. Quercetin protects rat BMSCs from oxidative stress via ferroptosis. J Mol Endocrinol. 2022;69(3):401–13. https://doi.org/10.1530/jme-22-0086.
Egert S, Wolffram S, Bosy-Westphal A, Boesch-Saadatmandi C, Wagner AE, Frank J, et al. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J Nutr. 2008;138(9):1615–21. https://doi.org/10.1093/jn/138.9.1615.
Lin Y, Kazlova V, Ramakrishnan S, Murray MA, Fast D, Chandra A, et al. Bone health nutraceuticals alter microarray mRNA gene expression: a randomized, parallel, open-label clinical study. Phytomedicine. 2016;23(1):18–26. https://doi.org/10.1016/j.phymed.2015.11.011.
Lee S, Park H, Oh JS, Byun K, Kim DY, Yun HS, et al. Hydroxyapatite microbeads containing BMP-2 and quercetin fabricated via electrostatic spraying to encourage bone regeneration. Biomed Eng Online. 2023;22(1):15. https://doi.org/10.1186/s12938-023-01078-y.
Attar ES, Chaudhari VH, Deokar CG, Dyawanapelly S, Devarajan PV. Nano drug delivery strategies for an oral bioenhanced quercetin formulation. Eur J Drug Metab Pharmacokinet. 2023;48(5):495–514. https://doi.org/10.1007/s13318-023-00843-7.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 82104730). The 72nd Batch of China Postdoctoral Science Foundation (2022M721065) and Central Plains Talent Program-Science and Technology Innovation Leading Talent Project (224200510027).
Author information
Authors and Affiliations
Contributions
Feng, Dang, and Liu wrote the main manuscript text. Feng and Liu prepared Fig. 1 and Table 1. Lin, Nie, and Liao conducted the literature search and collation. Zheng, Che, and Yao revised the manuscript text. Liu and Zhang provided the idea and funding. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanchen Feng and Xue Dang share the first authorship; both authors are involved equally in the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, Y., Dang, X., Zheng, P. et al. Quercetin in Osteoporosis Treatment: A Comprehensive Review of Its Mechanisms and Therapeutic Potential. Curr Osteoporos Rep (2024). https://doi.org/10.1007/s11914-024-00868-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s11914-024-00868-0