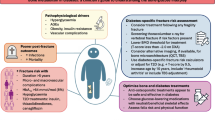
Abstract
Purpose of Review
Bone marrow adipose tissue (BMAT) in the skeleton likely plays a variety of physiological and pathophysiological roles that are not yet fully understood. In elucidating the complex relationship between bone and BMAT, glucocorticoids (GCs) are positioned to play a key role, as they have been implicated in the differentiation of bone marrow mesenchymal stem cells (BMSCs) between osteogenic and adipogenic lineages. The purpose of this review is to illuminate aspects of both endogenous and exogenous GC signaling, including the influence of GC receptors, in mechanisms of bone aging including relationships to BMAT.
Recent Findings
Harmful effects of GCs on bone mass involve several cellular pathways and events that can include BMSC differentiation bias toward adipogenesis and the influence of mature BMAT on bone remodeling through crosstalk. Interestingly, BMAT involvement remains poorly explored in GC-induced osteoporosis and warrants further investigation.
Summary
This review provides an update on the current understanding of the role of glucocorticoids in the biology of osteoblasts and bone marrow adipocytes (BMAds).

Similar content being viewed by others
References
Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018;28:436–53.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The Hallmarks of Aging. Cell. 2013;153:1194–217.
Lecot P, Alimirah F, Desprez PY, Campisi J, Wiley C. Context-dependent effects of cellular senescence in cancer development. Br J Cancer. 2016;114:1180–4.
Macías I, Alcorta-Sevillano N, Rodríguez CI, Infante A. Osteoporosis and the Potential of Cell-Based Therapeutic Strategies. Int J Mol Sci. 2020;21:1653.
Zhang Q, Cai W, Wang G, Shen X. Prevalence and contributing factors of osteoporosis in the elderly over 70 years old: an epidemiological study of several community health centers in Shanghai. Ann Palliat Med. 2020;9:231–8.
Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–25.
Russell RGG, Espina B, Hulley P. Bone biology and the pathogenesis of osteoporosis. Curr Opin Rheumatol. 2006;18:S3–S10.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science. 1999;284:143–7.
Sharma AK, Shi X, Isales CM, McGee-Lawrence ME. Endogenous Glucocorticoid Signaling in the Regulation of Bone and Marrow Adiposity: Lessons from Metabolism and Cross Talk in Other Tissues. Curr Osteoporos Rep. 2019;17:438–45.
Paspaliaris V, Kolios G. Stem cells in Osteoporosis: From Biology to New Therapeutic Approaches. Stem Cells Int. 2019;2019:1730978–16.
Han L, Wang B, Wang R, Gong S, Chen G, Xu W. The shift in the balance between osteoblastogenesis and adipogenesis of mesenchymal stem cells mediated by glucocorticoid receptor. Stem Cell Res Ther. 2019;10:377.
Li J, Zhang N, Huang X, Xu J, Fernandes JC, Dai K, Zhang X. Dexamethasone shifts bone marrow stromal cells from osteoblasts to adipocytes by C/EBPalpha promoter methylation. Cell Death Dis. 2013;4:e832–2.
Lecka-Czernik B, Rosen CJ, Kawai M. Skeletal aging and the adipocyte program: New insights from an "old" molecule. Cell Cycle. 2010;9:3648–54.
Veldhuis-Vlug AG, Rosen CJ. Clinical implications of bone marrow adiposity. J Intern Med. 2018;283:121–39.
Bethel M, Chitteti BR, Srour EF, Kacena MA. The changing balance between osteoblastogenesis and adipogenesis in aging and its impact on hematopoiesis. Curr Osteoporos Rep. 2013;11:99–106.
McGee-Lawrence ME, Carpio LR, Schulze RJ, Pierce JL, McNiven MA, Farr JN, Khosla S, Oursler MJ, Westendorf JJ. Hdac3 Deficiency Increases Marrow Adiposity and Induces Lipid Storage and Glucocorticoid Metabolism in Osteochondroprogenitor Cells. J Bone Mineral Res Official J Am Soc Bone Mineral Res. 2016;31:116–28.
Razidlo DF, Whitney TJ, Casper ME, McGee-Lawrence ME, Stensgard BA, Li X, Secreto FJ, Knutson SK, Hiebert SW, Westendorf JJ. Histone deacetylase 3 depletion in osteo/chondroprogenitor cells decreases bone density and increases marrow fat. PLoS One. 2010;5:e11492.
Devlin MJ, Rosen CJ. The bone–fat interface: basic and clinical implications of marrow adiposity. Lancet Diab Endocrinol. 2015;3:141–7.
Tikhonova AN, Dolgalev I, Hu H, Sivaraj KK, Hoxha E, Cuesta-Domínguez Á, Pinho S, Akhmetzyanova I, Gao J, Witkowski M, Guillamot M, Gutkin MC, Zhang Y, Marier C, Diefenbach C, Kousteni S, Heguy A, Zhong H, Fooksman DR, et al. The bone marrow microenvironment at single-cell resolution. Nature. 2019;569:222–8.
Baryawno N, Przybylski D, Kowalczyk MS, Kfoury Y, Severe N, Gustafsson K, Kokkaliaris KD, Mercier F, Tabaka M, Hofree M, Dionne D, Papazian A, Lee D, Ashenberg O, Subramanian A, Vaishnav ED, Rozenblatt-Rosen O, Regev A, Scadden DT. A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell. 2019;177:1915–1932.e1916.
Wolock SL, Krishnan I, Tenen DE, Matkins V, Camacho V, Patel S, Agarwal P, Bhatia R, Tenen DG, Klein AM, Welner RS. Mapping Distinct Bone Marrow Niche Populations and Their Differentiation Paths. Cell Rep. 2019;28:302–311.e305.
Dolgalev, I., and Tikhonova, A. N. Connecting the Dots: Resolving the Bone Marrow Niche Heterogeneity. Front Cell Dev Biol. 2021 Mar 12;9:622519. https://doi.org/10.3389/fcell.2021.622519
Matsushita, Y., Ono, W., and Ono, N. Toward Marrow Adipocytes: Adipogenic Trajectory of the Bone Marrow Stromal Cell Lineage. Front Endocrinol. 2022 Apr 22;13:882297. https://doi.org/10.3389/fendo.2022.882297
Trudel G, Payne M, Mädler B, Ramachandran N, Lecompte M, Wade C, Biolo G, Blanc S, Hughson R, Bear L, Uhthoff HK. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. J Appl Physiol. 2009;107:540–8.
Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18:641–7.
Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome DT, Soliman SS, DelProposto JL, Lumeng CN, Mitra A, Pandit SV, Gallagher KA, Miller JD, Krishnan V, Hui SK, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–75.
Suchacki KJ, Tavares AAS, Mattiucci D, Scheller EL, Papanastasiou G, Gray C, Sinton MC, Ramage LE, McDougald WA, Lovdel A, Sulston RJ, Thomas BJ, Nicholson BM, Drake AJ, Alcaide-Corral CJ, Said D, Poloni A, Cinti S, Macpherson GJ, et al. Bone marrow adipose tissue is a unique adipose subtype with distinct roles in glucose homeostasis. Nat Commun. 2020;11:3097.
Li, Z., Bowers, E., Zhu, J., Yu, H., Hardij, J., Bagchi, D. P., Mori, H., Lewis, K. T., Granger, K., Schill, R. L., Romanelli, S. M., Abrishami, S., Hankenson, K. D., Singer, K., Rosen, C. J., and MacDougald, O. A. Lipolysis of bone marrow adipocytes is required to fuel bone and the marrow niche during energy deficits Elife. 2022 Jun 22;11:e78496. https://doi.org/10.7554/eLife.78496
Schwartz AV, Sigurdsson S, Hue TF, Lang TF, Harris TB, Rosen CJ, Vittinghoff E, Siggeirsdottir K, Sigurdsson G, Oskarsdottir D, Shet K, Palermo L, Gudnason V, Li X. Vertebral Bone Marrow Fat Associated With Lower Trabecular BMD and Prevalent Vertebral Fracture in Older Adults. J Clin Endocrinol Metab. 2013;98:2294–300.
Chandra A, Lagnado AB, Farr JN, Schleusner M, Monroe DG, Saul D, Passos JF, Khosla S, Pignolo RJ. Bone Marrow Adiposity in Models of Radiation- and Aging-Related Bone Loss Is Dependent on Cellular Senescence. J Bone Miner Res. 2022;37:997–1011.
Li J, Lu L, Liu Y, Yu X. Bone marrow adiposity during pathologic bone loss: molecular mechanisms underlying the cellular events. J Mol Med. 2022;100:167–83.
Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. Increased Bone Marrow Fat in Anorexia Nervosa. J Clin Endocrinol Metab. 2009;94:2129–36.
Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Mineral Res Official J Am Soc Bone Mineral Res. 2010;25:2078–88.
Baum T, Yap SP, Karampinos DC, Nardo L, Kuo D, Burghardt AJ, Masharani UB, Schwartz AV, Li X, Link TM. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J Magn Reson Imaging. 2012;35:117–24.
Gorgey AS, Poarch HJ, Adler RA, Khalil RE, Gater DR. Femoral bone marrow adiposity and cortical bone cross-sectional areas in men with motor complete spinal cord injury. PM & R : J Injury, Funct Rehab. 2013;5:939–48.
Esche J, Shi L, Hartmann MF, Schonau E, Wudy SA, Remer T. Glucocorticoids and Body Fat Inversely Associate With Bone Marrow Density of the Distal Radius in Healthy Youths. J Clin Endocrinol Metab. 2019;104:2250–6.
Vande Berg BC, Malghem J, Lecouvet FE, Devogelaer JP, Maldague B, Houssiau FA. Fat conversion of femoral marrow in glucocorticoid-treated patients: A cross-sectional and longitudinal study with magnetic resonance imaging. Arthritis Rheum. 1999;42:1405–11.
Li Z, MacDougald OA. Preclinical models for investigating how bone marrow adipocytes influence bone and hematopoietic cellularity. Best Pract Res Clin Endocrinol Metab. 2021;35:101547.
Pierce JL, Sharma AK, Roberts RL, Yu K, Irsik DL, Choudhary V, Dorn JS, Bensreti H, Benson RD Jr, Kaiser H, Khayrullin A, Davis C, Wehrle CJ, Johnson MH, Bollag WB, Hamrick MW, Shi X, Isales CM, McGee-Lawrence ME. The Glucocorticoid Receptor in Osterix-Expressing Cells Regulates Bone Mass, Bone Marrow Adipose Tissue, and Systemic Metabolism in Female Mice During Aging. J Bone Miner Res. 2022;37:285–302.
Romacho T, Elsen M, Röhrborn D, Eckel J. Adipose tissue and its role in organ crosstalk. Acta Physiol (Oxford). 2014;210:733–53.
Maurin AC, Chavassieux PM, Frappart L, Delmas PD, Serre CM, Meunier PJ. Influence of mature adipocytes on osteoblast proliferation in human primary cocultures. Bone. 2000;26:485–9.
Iwaniec UT, Turner RT. Failure to generate bone marrow adipocytes does not protect mice from ovariectomy-induced osteopenia. Bone. 2013;53:145–53.
Botolin S, McCabe LR. Inhibition of PPARgamma prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol. 2006;209:967–76.
Almeida M, Kim HN, Han L, Zhou D, Thostenson J, Porter RM, Ambrogini E, Manolagas SC, Jilka RL. Increased marrow adipogenesis does not contribute to age-dependent appendicular bone loss in female mice. Aging Cell. 2020;19:e13247.
Cawthorn WP, Scheller EL, Parlee SD, Pham HA, Learman BS, Redshaw CM, Sulston RJ, Burr AA, Das AK, Simon BR, Mori H, Bree AJ, Schell B, Krishnan V, MacDougald OA. Expansion of Bone Marrow Adipose Tissue During Caloric Restriction Is Associated With Increased Circulating Glucocorticoids and Not With Hypoleptinemia. Endocrinology. 2016;157:508–21.
Zhou H, Cooper MS, Seibel MJ. Endogenous Glucocorticoids and Bone. Bone Res. 2013;1:107–19.
Buttgereit F, Burmester GR, Straub RH, Seibel MJ, Zhou H. Exogenous and endogenous glucocorticoids in rheumatic diseases. Arthritis Rheum. 2011;63:1–9.
Meikle AW, Tyler FH. Potency and duration of action of glucocorticoids. Effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. Am J Med. 1977;63:200–7.
Parente L. Deflazacort: therapeutic index, relative potency and equivalent doses versus other corticosteroids. BMC Pharmacol Toxicol. 2017;18:1.
Caplan A, Fett N, Rosenbach M, Werth VP, Micheletti RG. Prevention and management of glucocorticoid-induced side effects: A comprehensive review: A review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76:1–9.
Zoorob RJ, Cender D. A different look at corticosteroids. Am Fam Physician. 1998;58:443–50.
Li, Z., Liu, C., Li, S., Li, T., Li, Y., Wang, N., Bao, X., Xue, P., and Liu, S. (2021) BMSC-Derived Exosomes Inhibit Dexamethasone-Induced Muscle Atrophy via the miR-486-5p/FoxO1 Axis. Front Endocrinol (Lausanne). 2021 Oct 1;12:681267. https://doi.org/10.3389/fendo.2021.681267
Tauchmanova L, Pivonello R, Di Somma C, Rossi R, De Martino MC, Camera L, Klain M, Salvatore M, Lombardi G, Colao A. Bone demineralization and vertebral fractures in endogenous cortisol excess: role of disease etiology and gonadal status. J Clin Endocrinol Metab. 2006;91:1779–84.
O'Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–41.
Sui B, Hu C, Liao L, Chen Y, Zhang X, Fu X, Zheng C, Li M, Wu L, Zhao X, Jin Y. Mesenchymal progenitors in osteopenias of diverse pathologies: differential characteristics in the common shift from osteoblastogenesis to adipogenesis. Sci Rep. 2016;6:30186.
Rogatsky I, Trowbridge JM, Garabedian MJ. Glucocorticoid receptor-mediated cell cycle arrest is achieved through distinct cell-specific transcriptional regulatory mechanisms. Mol Cell Biol. 1997;17:3181–93.
Chang JK, Li CJ, Liao HJ, Wang CK, Wang GJ, Ho ML. Anti-inflammatory drugs suppress proliferation and induce apoptosis through altering expressions of cell cycle regulators and pro-apoptotic factors in cultured human osteoblasts. Toxicology. 2009;258:148–56.
Li H, Qian W, Weng X, Wu Z, Li H, Zhuang Q, Feng B, Bian Y. Glucocorticoid receptor and sequential P53 activation by dexamethasone mediates apoptosis and cell cycle arrest of osteoblastic MC3T3-E1 cells. PLoS One. 2012;7:e37030.
Gado M, Baschant U, Hofbauer LC, Henneicke H. Bad to the Bone: The Effects of Therapeutic Glucocorticoids on Osteoblasts and Osteocytes. Front Endocrinol (Lausanne). 2022;13:835720.
Lane NE, Yao W, Balooch M, Nalla RK, Balooch G, Habelitz S, Kinney JH, Bonewald LF. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Mineral Res Official J Am Soc Bone Mineral Res. 2006;21:466–76.
Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–82.
Sato AY, Tu X, McAndrews KA, Plotkin LI, Bellido T. Prevention of glucocorticoid induced-apoptosis of osteoblasts and osteocytes by protecting against endoplasmic reticulum (ER) stress in vitro and in vivo in female mice. Bone. 2015;73:60–8.
Jia J, Yao W, Guan M, Dai W, Shahnazari M, Kar R, Bonewald L, Jiang JX, Lane NE. Glucocorticoid dose determines osteocyte cell fate. FASEB J. 2011;25:3366–76.
Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, Kirilov M, Mandic V, Takacz A, Schmidt-Ullrich R, Ostermay S, Schinke T, Spanbroek R, Zaiss MM, Angel PE, Lerner UH, David JP, Reichardt HM, Amling M, et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010;11:517–31.
Zhang S, Liu Y, Liang Q. Low-dose dexamethasone affects osteoblast viability by inducing autophagy via intracellular ROS. Mol Med Rep. 2018;17:4307–16.
Yao W, Dai W, Jiang L, Lay EY, Zhong Z, Ritchie RO, Li X, Ke H, Lane NE. Sclerostin-antibody treatment of glucocorticoid-induced osteoporosis maintained bone mass and strength. Osteoporosis International : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2016;27:283–94.
Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–9.
Fairfield H, Falank C, Harris E, Demambro V, McDonald M, Pettitt JA, Mohanty ST, Croucher P, Kramer I, Kneissel M, Rosen CJ, Reagan MR. The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis. J Cell Physiol. 2018;233:1156–67.
Kim SP, Da H, Wang L, Taketo MM, Wan M, Riddle RC. Bone-derived sclerostin and Wnt/beta-catenin signaling regulate PDGFRalpha(+) adipoprogenitor cell differentiation. FASEB J. 2021;35:e21957.
Balani DH, Trinh S, Xu M, Kronenberg HM. Sclerostin Antibody Administration Increases the Numbers of Sox9creER+ Skeletal Precursors and Their Progeny. J Bone Miner Res. 2021;36:757–67.
Gao J, Cheng TS, Qin A, Pavlos NJ, Wang T, Song K, Wang Y, Chen L, Zhou L, Jiang Q, Takayanagi H, Yan S, Zheng M. Glucocorticoid impairs cell-cell communication by autophagy-mediated degradation of connexin 43 in osteocytes. Oncotarget. 2016;7:26966–78.
Abdallah BM, Kassem M. New factors controlling the balance between osteoblastogenesis and adipogenesis. Bone. 2012;50:540–5.
Maurin AC, Chavassieux PM, Vericel E, Meunier PJ. Role of polyunsaturated fatty acids in the inhibitory effect of human adipocytes on osteoblastic proliferation. Bone. 2002;31:260–6.
Wang D, Haile A, Jones LC. Dexamethasone-induced lipolysis increases the adverse effect of adipocytes on osteoblasts using cells derived from human mesenchymal stem cells. Bone. 2013;53:520–30.
Elbaz A, Wu X, Rivas D, Gimble JM, Duque G. Inhibition of fatty acid biosynthesis prevents adipocyte lipotoxicity on human osteoblasts in vitro. J Cell Mol Med. 2010;14:982–91.
Martel D, Leporq B, Saxena A, Belmont HM, Turyan G, Honig S, Regatte RR, Chang G. 3T chemical shift-encoded MRI: Detection of altered proximal femur marrow adipose tissue composition in glucocorticoid users and validation with magnetic resonance spectroscopy. J Magn Reson Imaging. 2019;50:490–6.
Liu K, Jing Y, Zhang W, Fu X, Zhao H, Zhou X, Tao Y, Yang H, Zhang Y, Zen K, Zhang C, Li D, Shi Q. Silencing miR-106b accelerates osteogenesis of mesenchymal stem cells and rescues against glucocorticoid-induced osteoporosis by targeting BMP2. Bone. 2017;97:130–8.
Li CJ, Cheng P, Liang MK, Chen YS, Lu Q, Wang JY, Xia ZY, Zhou HD, Cao X, Xie H, Liao EY, Luo XH. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest. 2015;125:1509–22.
Kang H, Chen H, Huang P, Qi J, Qian N, Deng L, Guo L. Glucocorticoids impair bone formation of bone marrow stromal stem cells by reciprocally regulating microRNA-34a-5p. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2016;27:1493–505.
Wang G, Wang F, Zhang L, Yan C, Zhang Y. miR-133a silencing rescues glucocorticoid-induced bone loss by regulating the MAPK/ERK signaling pathway. Stem Cell Res Ther. 2021;12:215.
Shen GY, Ren H, Shang Q, Zhao WH, Zhang ZD, Yu X, Huang JJ, Tang JJ, Yang ZD, Liang D, Jiang XB. Let-7f-5p regulates TGFBR1 in glucocorticoid-inhibited osteoblast differentiation and ameliorates glucocorticoid-induced bone loss. Int J Biol Sci. 2019;15:2182–97.
Kalluri R, LeBleu Valerie S. The biology, function, and biomedical applications of exosomes. Sci. 2020;367:eaau6977.
Liu Y, Wang C, Wei M, Yang G, Yuan L. Multifaceted Roles of Adipose Tissue-Derived Exosomes in Physiological and Pathological Conditions. Front Physiol. 2021;12:669429.
Quan M, Kuang S. Exosomal Secretion of Adipose Tissue during Various Physiological States. Pharm Res. 2020;37:221.
Tao SC, Yuan T, Rui BY, Zhu ZZ, Guo SC, Zhang CQ. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics. 2017;7:733–50.
Nan K, Zhang Y, Zhang X, Li D, Zhao Y, Jing Z, Liu K, Shang D, Geng Z, Fan L. Exosomes from miRNA-378-modified adipose-derived stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head by enhancing angiogenesis and osteogenesis via targeting miR-378 negatively regulated suppressor of fused (Sufu). Stem Cell Res Ther. 2021;12:331.
Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol Sci. 2013;34:518–30.
Fudulu DP, Horn G, Hazell G, Lefrançois-Martinez AM, Martinez A, Angelini GD, Lightman SL, Spiga F. Co-culture of monocytes and zona fasciculata adrenal cells: An in vitro model to study the immune-adrenal cross-talk. Mol Cell Endocrinol. 2021;526:111195.
Pon LA, Hartigan JA, Orme-Johnson NR. Acute ACTH regulation of adrenal corticosteroid biosynthesis. Rapid Accum Phosphoprotein J Biol Chem. 1986;261:13309–16.
Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, Hewison M, Stewart PM. 11beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25:831–66.
Almanzar G, Mayerl C, Seitz JC, Hofner K, Brunner A, Wild V, Jahn D, Geier A, Fassnacht M, Prelog M. Expression of 11beta-hydroxysteroid-dehydrogenase type 2 in human thymus. Steroids. 2016;110:35–40.
Albiston AL, Obeyesekere VR, Smith RE, Krozowski ZS. Cloning and tissue distribution of the human 11 beta-hydroxysteroid dehydrogenase type 2 enzyme. Mol Cell Endocrinol. 1994;105:R11–7.
Luft FC. 11beta-Hydroxysteroid Dehydrogenase-2 and Salt-Sensitive Hypertension. Circulation. 2016;133:1335–7.
Salvante KG, Milano K, Kliman HJ, Nepomnaschy PA. Placental 11 beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2) expression very early during human pregnancy. J Dev Orig Health Dis. 2017;8:149–54.
Whorwood CB, Ricketts ML, Stewart PM. Epithelial cell localization of type 2 11 beta-hydroxysteroid dehydrogenase in rat and human colon. Endocrinology. 1994;135:2533–41.
Justesen J, Mosekilde L, Holmes M, Stenderup K, Gasser J r, Mullins JJ, Seckl JR, Kassem M. Mice Deficient in 11β-Hydroxysteroid Dehydrogenase Type 1 Lack Bone Marrow Adipocytes, but Maintain Normal Bone Formation. Endocrinology. 2004;145:1916–25.
Boucher E, Provost PR, Tremblay Y. Ontogeny of adrenal-like glucocorticoid synthesis pathway and of 20alpha-hydroxysteroid dehydrogenase in the mouse lung. BMC Res Notes. 2014;7:119.
Chapman K, Holmes M, Seckl J. 11β-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev. 2013;93:1139–206.
Gilmour JS, Coutinho AE, Cailhier JF, Man TY, Clay M, Thomas G, Harris HJ, Mullins JJ, Seckl JR, Savill JS, Chapman KE. Local amplification of glucocorticoids by 11 beta-hydroxysteroid dehydrogenase type 1 promotes macrophage phagocytosis of apoptotic leukocytes. J Immunol. 2006;176:7605–11.
Ramamoorthy S, Cidlowski JA. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum Dis Clin N Am. 2016;42(15-31):vii.
Sher LB, Woitge HW, Adams DJ, Gronowicz GA, Krozowski Z, Harrison JR, Kream BE. Transgenic expression of 11beta-hydroxysteroid dehydrogenase type 2 in osteoblasts reveals an anabolic role for endogenous glucocorticoids in bone. Endocrinology. 2004;145:922–9.
Zhou H, Mak W, Kalak R, Street J, Fong-Yee C, Zheng Y, Dunstan CR, Seibel MJ. Glucocorticoid-dependent Wnt signaling by mature osteoblasts is a key regulator of cranial skeletal development in mice. Development. 2009;136:427–36.
Kalak R, Zhou H, Street J, Day RE, Modzelewski JR, Spies CM, Liu PY, Li G, Dunstan CR, Seibel MJ. Endogenous glucocorticoid signalling in osteoblasts is necessary to maintain normal bone structure in mice. Bone. 2009;45:61–7.
Yang M, Trettel LB, Adams DJ, Harrison JR, Canalis E, Kream BE. Col3.6-HSD2 transgenic mice: a glucocorticoid loss-of-function model spanning early and late osteoblast differentiation. Bone. 2010;47:573–82.
Sher LB, Harrison JR, Adams DJ, Kream BE. Impaired cortical bone acquisition and osteoblast differentiation in mice with osteoblast-targeted disruption of glucocorticoid signaling. Calcif Tissue Int. 2006;79:118–25.
Zhou H, Mak W, Zheng Y, Dunstan CR, Seibel MJ. Osteoblasts directly control lineage commitment of mesenchymal progenitor cells through Wnt signaling. J Biol Chem. 2008;283:1936–45.
Bentley L, Esapa CT, Nesbit MA, Head RA, Evans H, Lath D, Scudamore CL, Hough TA, Podrini C, Hannan FM, Fraser WD, Croucher PI, Brown MA, Brown SD, Cox RD, Thakker RV. An N-ethyl-N-nitrosourea induced corticotropin-releasing hormone promoter mutation provides a mouse model for endogenous glucocorticoid excess. Endocrinology. 2014;155:908–22.
Belaya ZE, Grebennikova TA, Melnichenko GA, Nikitin AG, Solodovnikov AG, Brovkina OI, Grigoriev AU, Rozhinskaya LY, Dedov II. Effects of endogenous hypercortisolism on bone mRNA and microRNA expression in humans. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2018;29:211–21.
Vestergaard P, Lindholm J, Jorgensen JO, Hagen C, Hoeck HC, Laurberg P, Rejnmark L, Brixen K, Kristensen LO, Feldt-Rasmussen U, Mosekilde L. Increased risk of osteoporotic fractures in patients with Cushing’s syndrome. Eur J Endocrinol. 2002;146:51–6.
Hartmann K, Koenen M, Schauer S, Wittig-Blaich S, Ahmad M, Baschant U, Tuckermann JP. Molecular Actions of Glucocorticoids in Cartilage and Bone During Health, Disease, and Steroid Therapy. Physiol Rev. 2016;96:409–47.
Zhao ZY, Lu FH, Xie Y, Fu YR, Bogdan A, Touitou Y. Cortisol secretion in the elderly. Influence of age, sex and cardiovascular disease in a Chinese population. Steroids. 2003;68:551–5.
Reynolds RM, Dennison EM, Walker BR, Syddall HE, Wood PJ, Andrew R, Phillips DI, Cooper C. Cortisol secretion and rate of bone loss in a population-based cohort of elderly men and women. Calcif Tissue Int. 2005;77:134–8.
Johar H, Emeny RT, Bidlingmaier M, Reincke M, Thorand B, Peters A, Heier M, Ladwig KH. Blunted diurnal cortisol pattern is associated with frailty: a cross-sectional study of 745 participants aged 65 to 90 years. J Clin Endocrinol Metab. 2014;99:E464–8.
Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O'Brien CA, Thostenson J, Roberson PK, Boskey AL, Clemens TL, Manolagas SC. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell. 2010;9:147–61.
Henneicke H, Kim S, Swarbrick MM, Li J, Gasparini SJ, Thai J, Foong D, Cavanagh LL, Fong-Yee C, Karsten E, Lin RCY, Cooper MS, Zhou H, Seibel MJ. Skeletal glucocorticoid signalling determines leptin resistance and obesity in aging mice. Mol Metabol. 2020;42:101098.
Vandewalle J, Luypaert A, De Bosscher K, Libert C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol Metab. 2018;29:42–54.
Timmermans, S., Souffriau, J., and Libert, C. (2019) A General Introduction to Glucocorticoid Biology. Front Immunol. 2019 Jul 4;10:1545. https://doi.org/10.3389/fimmu.2019.01545
Ratman D, Vanden Berghe W, Dejager L, Libert C, Tavernier J, Beck IM, De Bosscher K. How glucocorticoid receptors modulate the activity of other transcription factors: a scope beyond tethering. Mol Cell Endocrinol. 2013;380:41–54.
Porter, B. A., Ortiz, M. A., Bratslavsky, G., and Kotula, L. (2019) Structure and Function of the Nuclear Receptor Superfamily and Current Targeted Therapies of Prostate Cancer. Cancers (Basel). 2019 Nov 23;11(12):1852. https://doi.org/10.3390/cancers11121852
Mitsis T, Papageorgiou L, Efthimiadou A, Bacopoulou F, Vlachakis D, Chrousos GP, Eliopoulos E, Mitsis T, Papageorgiou L, Efthimiadou A, Bacopoulou F, Vlachakis D, Chrousos GP, Eliopoulos E. A comprehensive structural and functional analysis of the ligand binding domain of the nuclear receptor superfamily reveals highly conserved signaling motifs and two distinct canonical forms through evolution. World Acad Sci J. 2019;1:264–74.
Meijer OC, Buurstede JC, Schaaf MJM. Corticosteroid Receptors in the Brain: Transcriptional Mechanisms for Specificity and Context-Dependent Effects. Cell Mol Neurobiol. 2019;39:539–49.
Koning A, Buurstede JC, van Weert L, Meijer OC. Glucocorticoid and Mineralocorticoid Receptors in the Brain: A Transcriptional Perspective. J Endocr Soc. 2019;3:1917–30.
Rapp AE, Hachemi Y, Kemmler J, Koenen M, Tuckermann J, Ignatius A. Induced global deletion of glucocorticoid receptor impairs fracture healing. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2018;32:2235–45.
Pierce, J. L., Ding, K. H., Xu, J., Sharma, A. K., Yu, K., Del Mazo Arbona, N., Rodriguez-Santos, Z., Bernard, P., Bollag, W. B., Johnson, M. H., Hamrick, M. W., Begun, D. L., Shi, X. M., Isales, C. M., and McGee-Lawrence, M. E. (2019) The glucocorticoid receptor in osteoprogenitors regulates bone mass and marrow fat. J Endocrinol. 2019 Jul 1;JOE-19-0230.R1. https://doi.org/10.1530/JOE-19-0230
Fumoto T, Ishii KA, Ito M, Berger S, Schutz G, Ikeda K. Mineralocorticoid receptor function in bone metabolism and its role in glucocorticoid-induced osteopenia. Biochem Biophys Res Commun. 2014;447:407–12.
Castinetti F, Conte-Devolx B, Brue T. Medical Treatment of Cushing’s Syndrome: Glucocorticoid Receptor Antagonists and Mifepristone. Neuroendocrinology. 2010;92(suppl 1):125–30.
Mahajan DK, London SN. Mifepristone (RU486): a review. Fertil Steril. 1997;68:967–76.
Adashi EY, Rajan RS, O'Mahony DP, Cohen IG. The next two decades of mifepristone at FDA: History as destiny. Contraception. 2022;109:1–7.
Beaman J, Prifti C, Schwarz EB, Sobota M. Medication to Manage Abortion and Miscarriage. J Gen Intern Med. 2020;35:2398–405.
Molitch ME. Glucocorticoid receptor blockers. Pituitary. 2022;25:733–6.
Meijer OC, Koorneef LL, Kroon J. Glucocorticoid receptor modulators. Ann Endocrinol (Paris). 2018;79:107–11.
Spitz IM, Grunberg SM, Chabbert-Buffet N, Lindenberg T, Gelber H, Sitruk-Ware R. Management of patients receiving long-term treatment with mifepristone. Fertil Steril. 2005;84:1719–26.
Morice C, Baker DG, Patel MM, Nolen TL, Nowak K, Hirsch S, Kosten TR, Verrico CD. A randomized trial of safety and pharmacodynamic interactions between a selective glucocorticoid receptor antagonist, PT150, and ethanol in healthy volunteers. Sci Rep. 2021;11:9876.
Rocha SM, Fagre AC, Latham AS, Cummings JE, Aboellail TA, Reigan P, Aldaz DA, McDermott CP, Popichak KA, Kading RC, Schountz T, Theise ND, Slayden RA, Tjalkens RB. A Novel Glucocorticoid and Androgen Receptor Modulator Reduces Viral Entry and Innate Immune Inflammatory Responses in the Syrian Hamster Model of SARS-CoV-2 Infection. Front Immunol. 2022;13:811430.
Tatman P, Fringuello A, Graner M, Lillehei K, Parasido E, Albanese C, Tewari AK, Chakravarty D, Nair S, Theise N. Pan-cancer analysis to identify a novel class of glucocorticoid and androgen receptor antagonists with potent anti-tumor activity. J Clin Oncol. 2020;38:e15663–3.
Hunt HJ, Belanoff JK, Walters I, Gourdet B, Thomas J, Barton N, Unitt J, Phillips T, Swift D, Eaton E. Identification of the Clinical Candidate (R)-(1-(4-Fluorophenyl)-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4,4a,5,6,7,8-hexahydro-1H-pyrazolo[3,4-g]isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methanone (CORT125134): A Selective Glucocorticoid Receptor (GR) Antagonist. J Med Chem. 2017;60:3405–21.
Rew Y, Du X, Eksterowicz J, Zhou H, Jahchan N, Zhu L, Yan X, Kawai H, McGee LR, Medina JC, Huang T, Chen C, Zavorotinskaya T, Sutimantanapi D, Waszczuk J, Jackson E, Huang E, Ye Q, Fantin VR, Sun D. Discovery of a Potent and Selective Steroidal Glucocorticoid Receptor Antagonist (ORIC-101). J Med Chem. 2018;61:7767–84.
Khom S, Rodriguez L, Gandhi P, Kirson D, Bajo M, Oleata CS, Vendruscolo LF, Mason BJ, Roberto M. Alcohol dependence and withdrawal increase sensitivity of central amygdalar GABAergic synapses to the glucocorticoid receptor antagonist mifepristone in male rats. Neurobiol Dis. 2022;164:105610.
Buurstede JC, Umeoka EHL, da Silva MS, Krugers HJ, Joëls M, Meijer OC. Application of a pharmacological transcriptome filter identifies a shortlist of mouse glucocorticoid receptor target genes associated with memory consolidation. Neuropharmacology. 2022;216:109186.
Pivonello R, Bancos I, Feelders RA, Kargi AY, Kerr JM, Gordon MB, Mariash CN, Terzolo M, Ellison N, Moraitis AG. Relacorilant, a Selective Glucocorticoid Receptor Modulator, Induces Clinical Improvements in Patients With Cushing Syndrome: Results From A Prospective, Open-Label Phase 2 Study. Front Endocrinol (Lausanne). 2021;12:662865.
Leng Y, Sun Y, Huang W, Lv C, Cui J, Li T, Wang Y. Identification of dicyclohexyl phthalate as a glucocorticoid receptor antagonist by molecular docking and multiple in vitro methods. Mol Biol Rep. 2021;48:3145–54.
Xu X, Chen Y, Zhu D, Zhao T, Xu R, Wang J, Hu L, Shen X. FX5 as a non-steroidal GR antagonist improved glucose homeostasis in type 2 diabetic mice via GR/HNF4α/miR-122-5p pathway. Aging (Albany NY). 2020;13:2436–58.
Feger M, Ewendt F, Strotmann J, Schäffler H, Kempe-Teufel D, Glosse P, Stangl GI, Föller M. Glucocorticoids dexamethasone and prednisolone suppress fibroblast growth factor 23 (FGF23). J Mol Med (Berl). 2021;99:699–711.
Jacobson L, Ansari T, Potts J, McGuinness OP. Glucocorticoid-deficient corticotropin-releasing hormone knockout mice maintain glucose requirements but not autonomic responses during repeated hypoglycemia. Am J Physiol Endocrinol Metab. 2006;291:E15–22.
Li, Z., Bagchi, D. P., Zhu, J., Bowers, E., Yu, H., Hardij, J., Mori, H., Granger, K., Skjaerlund, J. D., Mandair, G. S., Abrishami, S., Singer, K., Hankenson, K. D., Rosen, C. J., and MacDougald, O. A. (2022) Constitutive bone marrow adipocytes suppress local bone formation. JCI Insight, Constitutive bone marrow adipocytes suppress local bone formation
Rendina-Ruedy E, Rosen CJ. Lipids in the Bone Marrow: An Evolving Perspective. Cell Metab. 2020 Feb 4;31(2):219–231. https://doi.org/10.1016/j.cmet.2019.09.015
Kim, S. P., Li, Z., Zoch, M. L., Frey, J. L., Bowman, C. E., Kushwaha, P., Ryan, K. A., Goh, B. C., Scafidi, S., Pickett, J. E., Faugere, M. C., Kershaw, E. E., Thorek, D. L. J., Clemens, T. L., Wolfgang, M. J., and Riddle, R. C. (2017) Fatty acid oxidation by the osteoblast is required for normal bone acquisition in a sex- and diet-dependent manner JCI Insight 2
Dirckx N, Moorer MC, Clemens TL, Riddle RC. The role of osteoblasts in energy homeostasis. Nat Rev Endocrinol. 2019 Nov;15(11):651–665. https://doi.org/10.1038/s41574-019-0246-y
Kushwaha P, Alekos NS, Kim SP, Li Z, Wolfgang MJ, Riddle RC. Mitochondrial fatty acid beta-oxidation is important for normal osteoclast formation in growing female mice. Front Physiol. 2022;13:997358.
Phinney DG. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem. 2012;113:2806–12.
Li J, Zuo B, Zhang L, Dai L, Zhang X. Osteoblast versus Adipocyte: Bone Marrow Microenvironment-Guided Epigenetic Control. Case Rep Orthop Res. 2018;1:2–18.
Flehr A, Källgård J, Alvén J, Lagerstrand K, Papalini E, Wheeler M, Vandenput L, Kahl F, Axelsson KF, Sundh D, Mysore RS, Lorentzon M. Development of a novel method to measure bone marrow fat fraction in older women using high-resolution peripheral quantitative computed tomography. Osteoporos Int. 2022;33:1545–56.
He HP, Gu S. The PPAR-γ/SFRP5/Wnt/β-catenin signal axis regulates the dexamethasone-induced osteoporosis. Cytokine. 2021;143:155488.
Wiper-Bergeron N, Wu D, Pope L, Schild-Poulter C, Haché RJ. Stimulation of preadipocyte differentiation by steroid through targeting of an HDAC1 complex. EMBO J. 2003;22:2135–45.
Song LN, Coghlan M, Gelmann EP. Antiandrogen effects of mifepristone on coactivator and corepressor interactions with the androgen receptor. Mol Endocrinol. 2004;18:70–85.
Mammi C, Marzolla V, Armani A, Feraco A, Antelmi A, Maslak E, Chlopicki S, Cinti F, Hunt H, Fabbri A, Caprio M. A novel combined glucocorticoid-mineralocorticoid receptor selective modulator markedly prevents weight gain and fat mass expansion in mice fed a high-fat diet. Int J Obes. 2016;40:964–72.
Funding
The authors are supported by funding provided by the National Institute on Aging (NIA P01 AG036675, R01 AG 067510 and T35 AG 067577).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of interest
The contents of this publication do not represent the views of the Department of Veterans Affairs or the US Government. The authors state that they do not have existing conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Bone Marrow and Adipose Tissue
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bensreti, H., Alhamad, D.W., Gonzalez, A.M. et al. Update on the Role of Glucocorticoid Signaling in Osteoblasts and Bone Marrow Adipocytes During Aging. Curr Osteoporos Rep 21, 32–44 (2023). https://doi.org/10.1007/s11914-022-00772-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-022-00772-5